
New Journal of Chemistry p. 4579 - 4589 (2018)
Update date:2022-08-17
Topics:
 Lone, Shabir H.
Lone, Shabir H.
 Bhat, Muzzaffar A.
Bhat, Muzzaffar A.
 Lone, Rayees A.
Lone, Rayees A.
 Jameel, Salman
Jameel, Salman
 Lone, Javeed A.
Lone, Javeed A.
 Bhat, Khursheed A.
Bhat, Khursheed A.
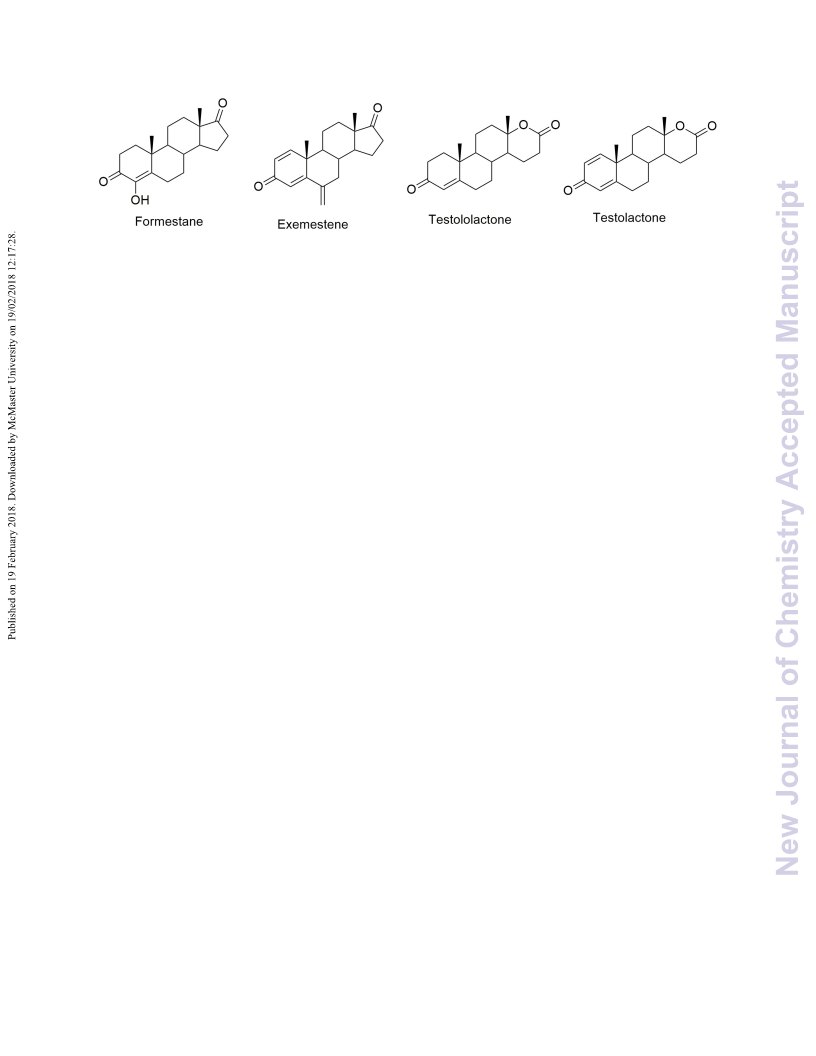
Testololactone (10) and testolactone (11) represent aromatase inhibitors containing lactone rings. We previously reported their hemisynthesis from the most common phytosterols, which are highly abundant in nature. Herein, we report the synthesis of their nitrogen congeners: testololactam (3) and testolactam (8). The reaction process involves the conversion of 4-androstene-3,17-dione to its corresponding oxime using hydroxylamine hydrochloride, whose Beckmann rearrangement under acid conditions yielded the desired testololactam (3). However, testolactam (8) was formed by the Beckmann rearrangement of the oxime (7) of 1,4-androstene-3,17-dienone (6). This expeditious reaction scheme may be exploited for the bulk production of testololactam (3) and testolactam (8). Theoretical DFT studies concerning the structural and electronic properties of all the end products were carried out using the Becke three-parameter Lee-Yang-Parr function (B3LYP) and 6-31G(d,p) level of theory. Molecular electrostatic potential map and frontier orbital analysis were carried out. The HOMO-LUMO energy gap was calculated, which allowed the calculation of relative reactivity descriptors like chemical hardness, chemical inertness, chemical potential, nucleophilicity and electrophilicity index of the synthesized products. The molecular docking studies of testololactam (3), testolactam (8) and testololactone (10), with aromatase (CYP19) revealed binding free energies of (ΔGb) = -9.85, -9.62 and -10.14 kcal mol-1 respectively, compared to the standard testolactone (11), a well-known aromatase inhibitor sold under the brand name TESLAC, which exhibited a binding free energy (ΔGb) of -10.29 kcal mol-1 with an inhibition constant Ki of 28.87 nM. The docking study revealed that the nitrogen congeners exhibit a relatively lower but appreciable therapeutic efficiency to be used as aromatase inhibitors.
View More




























Liaoyang hengye Chemical Co., Ltd.
Contact:86-419-5850866
Address:North Old Xiaoxiao Road,Yantai District, Dengta, Liaoyang, Liaoning, China
zhengzhou Triz Pharma-Tech Co., Ltd
website:http://www.Trizpharma-tech.com
Contact:+86-0371-86597269,53392065
Address:High-tech Industrial Development Zone, Zhengzhou City, NO.7 Holly Street
Hunan Dinuo Pharmaceutical Co.,Ltd.
Contact:86-731-88280100*8561
Address:Bio-pharmaceutical industrial park, Liuyang, Hunan, China
Changzhou Litong Chemical Co., Ltd.
website:http://www.litonchem.com/
Contact:+86-519-86301238
Address:Laoba Rd, Hutang town Changzhou Jiangsu
Anhui Jiatiansen Agrochemical Co.,Ltd
Contact:15366811918
Address:chemical industrial park,xiangyu town,dongzhi county,anhui,china
Doi:10.1021/jp0498318
(2004)Doi:10.1016/j.tet.2015.10.053
(2016)Doi:10.1039/b803828g
(2008)Doi:10.1016/S0020-1693(00)88992-6
(1978)Doi:10.1016/S0031-9422(98)00656-6
(1999)Doi:10.1038/183734a0
(1959)