
Bulletin of the Chemical Society of Japan p. 3295 - 3300 (1988)
Update date:2022-08-11
Topics:
 Matsui, Hiroshi
Matsui, Hiroshi
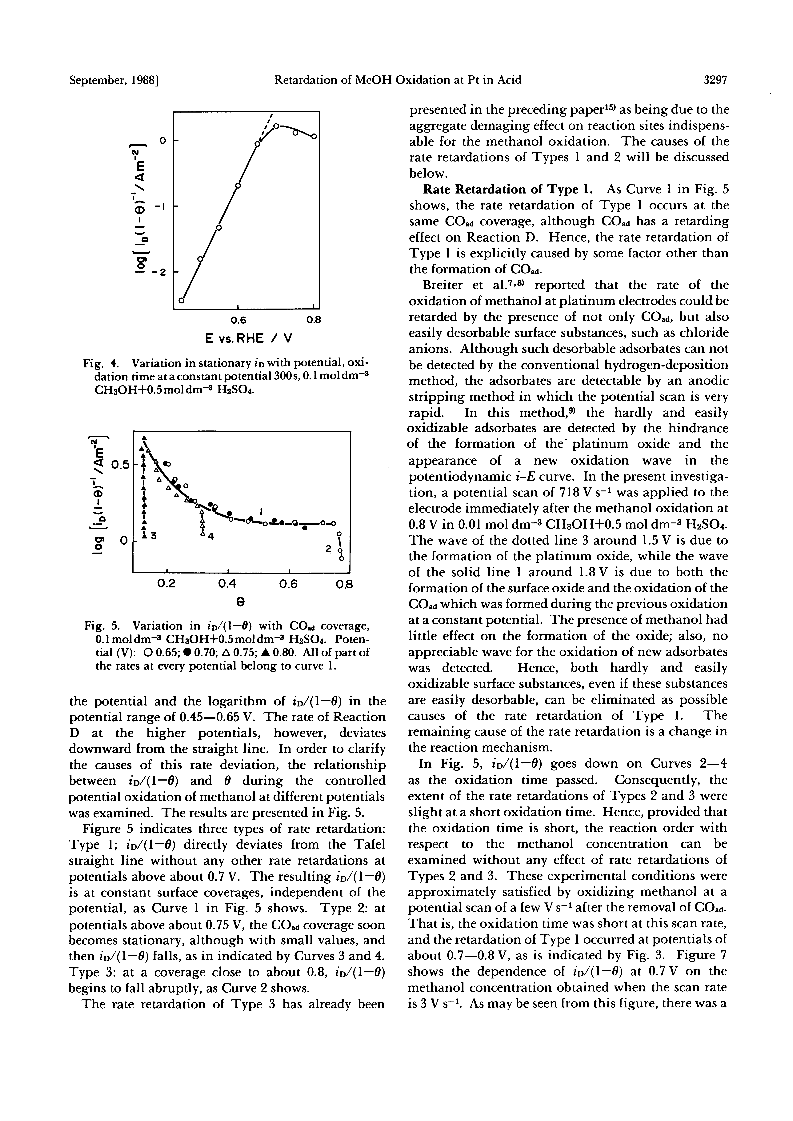
The rate retardation of the oxidation of methanol at the potential range of about 0.65-0.8 V vs. a reversible hydrogen electrode on a platinum electrode in 0.5 mol dm-3 H2SO4 was studied.The rate retardation of the overall oxidation was caused by that of the oxidation, Reaction D, not via COad.From the relationship among the rate of Reaction D, the COad coverage, and the potentials, three types of rate retardation were found out: Type 1-Reaction D is not accelerated by the potential, and the rate of the reaction is determined by the COad coverage and the methanol concentration.Type 2- the rate of Reaction D decreases at stationary COad coverages as the oxidation is prolonged.Type 3- the rate decreases at COad coverages close to the limiting value.It is proposed that Types 1 and 2 of the rate retardations take place when the adsorption of methanol molecules is rate-determining, and when the formaldehyde and formic acid formed from methanol are accumulated in the vicinity of the electrode, respectively.Type 3 of the rate retardation has been explained in a preceding paper in terms of the aggregate damaging effect of COad.
View More



Laohekou Jinghong Chemical Co.,Ltd
Contact:+86-0710-3702747
Address:163.East,Huagong Road,Laohekou
Shanghai Standard Biotech Co., Ltd.
Contact:+86-18502101150
Address:Room 103, Building 2nd, NO.720, Cailun Road , Pudong District, Shanghai, China
Daqing E-shine Chemical Co.,LTD
Contact:0086-024-31285112
Address:Hongweiyuan area, Ranghulu district
MedicalChem(Yancheng)Manuf.Co.,Ltd.
Contact:+86-515-84383366
Address:Touzeng BinHai, YanCheng City, JiangSu Province, China
Contact:+86-519-86623222
Address:29F/D, 99 Yanling West Road, Changzhou, Jiangsu, China
Doi:10.1016/0040-4039(95)00711-K
(1995)Doi:10.1038/s41557-019-0304-z
(2019)Doi:10.1039/P29760001342
(1976)Doi:10.3762/bjoc.13.98
(2017)Doi:10.1016/S0040-4039(00)70652-6
(1989)Doi:10.1021/jo00898a038
(1975)