- Lead(II) acetate
- Lead(II) arsenite
- Lead(II) azide
- Lead(II) bromide
- Lead(II) carbonate basic
- LEAD(II) CHLORITE
- Lead(II) cyanide
- Lead(II) diethyldithiocarbamate
- Lead(II) EDTA complex
- Lead(II) fluorosilicate
- 153483-31-92H-Cyclohepta[b]furan-2-one,6-[1-(acetyloxy)-3-oxobutyl]-3,3a,4,7,8,8a-hexahydro-7-methyl-3-methylene-,[3aR-[3aa,6(S*),7b,8ab]]- (9CI)
- 1534-90-3L-Phenylalanine,4-fluoro-, ethyl ester, hydrochloride (1:1)
- 153493-52-85,7-Dioxa-6-thiaspiro[2.5]octane, 6,6-dioxide
- 15350-13-7Copper, tetrakis[m-(benzoato-kO:kO')]di-, (Cu-Cu)
- 153504-70-2Spiro[1-azabicyclo[2.2.2]octane-3,5'-[1,3]oxathiolane],2'-methyl-, hydrochloride, hydrate (2:2:1), (2'R,3R)-rel-
- 153505-36-3Benzenamine,4-bromo-5-fluoro-2-nitro-
- 153505-37-41,2-Benzenediamine,4-bromo-5-fluoro-
- 153505-39-61,2-Benzenediamine,3,4-difluoro-
- 153506-14-0Acetamide,N-(5-chloro-2-hydroxy-4-methylphenyl)-
- 15351-09-41-Propanone,2-(dimethylamino)-1-phenyl-
Hot Products
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid

|
Basic Information |

|
Post buying leads |

|
Suppliers |

| Name |
Lead(II) acetate |
EINECS | 239-379-4 |
| CAS No. | 15347-57-6 | Density | g/cm3 |
| PSA | 37.30000 | LogP | -0.06490 |
| Solubility | N/A | Melting Point |
280ºC |
| Formula | C2H4 O2 . x Pb | Boiling Point | 117.1°Cat760mmHg |
| Molecular Weight | 1510.39 | Flash Point | 40°C |
| Transport Information | N/A | Appearance | N/A |
| Safety | A poison by intraperitoneal route. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Pb. | Risk Codes | N/A |
| Molecular Structure |
|
Hazard Symbols | 3 |
| Synonyms |
Leadacetate |
Lead(II) acetate Chemical Properties
Molecular Structure of Lead(II) acetate (CAS NO.15347-57-6):
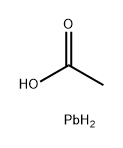
IUPAC Name: Lead(2+) diacetate
EINECS: 239-379-4
Molecular Formula: C4H6O4Pb
Molecular Weight: 325.288040 g/mol
H-Bond Donor: 0
H-Bond Acceptor: 4
Canonical SMILES: CC(=O)[O-].CC(=O)[O-].[Pb+2]
InChI: InChI=1S/2C2H4O2.Pb/c2*1-2(3)4;/h2*1H3,(H,3,4);/q;;+2/p-2
InChIKey: GUWSLQUAAYEZAF-UHFFFAOYSA-L
Product Categories: Organic-metal salt
Flash Point: 40 °C
Enthalpy of Vaporization: 23.7 kJ/mol
Boiling Point: 117.1 °C at 760 mmHg
Vapour Pressure: 13.9 mmHg at 25 °C
Lead(II) acetate Uses
Lead(II) acetate with cas registry number of 15347-57-6 is used as a reagent to make other lead compounds. Lead(II) acetate is also used as a mordant in textile printing and dyeing, as a drier in paints and varnishes. In low concentrations, it is the principal active ingredient in progressive types of hair coloring dyes. In ancient Romans, Lead(II) acetate can be used as a sugar substitute due to its sweet taste. But it is toxic and it is no longer used as a sweetener in most of the world because of its recognized toxicity. Lead(II) acetate has also been used in cosmetics throughout history. It is still used in men's hair coloring products like Grecian Formula. Lead acetate paper is used to detect the poisonous gas hydrogen sulfide. The gas reacts with lead(II) acetate on the moistened test paper to form a grey precipitate of lead(II) sulfide. An aqueous lead acetate solution is the byproduct of the 50/50 mixture of hydrogen peroxide and white vinegar. Lead acetate solution is used as folk remedy for sore nipples and used in the cleaning and maintenance of stainless steel firearm suppressors. Because of its high toxicity, this chemical solution must be appropriately disposed by a chemical processing facility or hazardous materials center. Alternately, the solution may be reacted with sulfuric acid to precipitate insoluble lead sulfate. The solid may then be removed by mechanical filtration and is safer to dispose of than aqueous lead acetate.
Lead(II) acetate Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 399mg/kg (399mg/kg) | Medycyna Pracy. Industrial Medicine. Vol. 26, Pg. 425, 1975. | |
| rat | LD50 | intraperitoneal | 286mg/kg (286mg/kg) | Neurobehavioral Toxicology and Teratology. Vol. 5, Pg. 91, 1983. |
Lead(II) acetate Safety Profile
A poison by intraperitoneal route. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Pb.
Lead(II) acetate Specification
Lead(II) acetate with cas registry number of 15347-57-6 is also called for Acetic acid, lead salt ; Lead acetate . It is a white crystalline substance with a sweetish taste, soluble in water and glycerin. It forms the trihydrate, Pb(CH3COO)2.3H2O, a colorless or white efflorescent monoclinic crystalline substance in water. Lead(II) acetate is prepared by treating litharge (lead(II) oxide, PbO) with acetic acid .

