
|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |
- Name Lopinavir
- EINECSN/A
- CAS No. 192725-17-0
- Density1.163 g/cm3
- PSA120.00000
- LogP4.39310
- SolubilityN/A
- Melting Point124-127 °C
- FormulaC37H48N4O5
- Boiling Point924.1 °C at 760 mmHg
- Molecular Weight628.812
- Flash Point512.7 °C
- Transport InformationN/A
- Appearancewhite crystalline solid
- Safety
- Risk CodesN/A
- Molecular Structure
- Hazard Symbols
 Xi
Xi - Synonyms
 Xi
Xi - Article Data20
Lopinavir Synthetic route

- 192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| In ethyl acetate at 25℃; for 8h; Solvent; | 97% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 25℃; for 8h; Solvent; | 96% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 96% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 96% |
- 192800-77-4
2S-(1-tetrahydro-pyrimid-2-onyl)-3-methyl butanoyl chloride

- 192725-49-8
(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane

- 192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| With 1H-imidazole In ethyl acetate; N,N-dimethyl-formamide at 0 - 20℃; Reagent/catalyst; Inert atmosphere; | 92% |
| With 1H-imidazole; dmap In ethyl acetate; N,N-dimethyl-formamide at 0 - 10℃; for 14h; |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 92% |

- 192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 92% |
- 192725-49-8
(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane

- 192725-50-1
(S)-tetrahydro-α-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetic acid

- 192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-amino-1,6-diphenylhexane; (S)-tetrahydro-α-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetic acid With thionyl chloride In dichloromethane at 0℃; for 2h; Reflux; Stage #2: With triethylamine In dichloromethane at 0 - 20℃; for 5h; | 90.1% |
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In N,N-dimethyl-formamide | 70% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 25℃; for 8h; Solvent; | 89% |

- 192725-17-0
lopinavir

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 25℃; for 8h; Solvent; | 89% |
Lopinavir Chemical Properties
IUPAC Name: (2S)-N-[(2S,4S,5S)-5-[[2-(2,6-Dimethylphenoxy)acetyl]amino]-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide
Formula: C37H48N4O5
Molecular Weight: 628.8 g/mol
Molecular Structure of Lopinavir (CAS NO.192725-17-0):
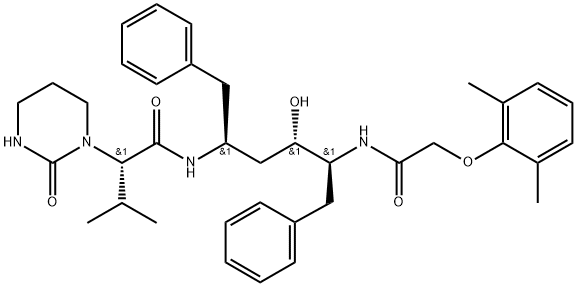
Melting Point: 124-127 °C
Flash Point: 512.7 °C
Boiling Point: 924.1 °C at 760 mmHg
Density: 1.163 g/cm3
Enthalpy of Vaporization: 140.81 kJ/mol
Appearance: white crystalline solid
Index of Refraction: 1.577
Molar Refractivity: 179.17 cm3
Molar Volume: 540.4 cm3
Surface Tension: 49.4 dyne/cm
XLogP3-AA: 5.9
H-Bond Donor: 4
H-Bond Acceptor: 5
Rotatable Bond Count: 15
Tautomer Count: 8
Exact Mass: 628.362471
MonoIsotopic Mass: 628.362471
Topological Polar Surface Area: 120
Heavy Atom Count: 46
Product Categories: Anti-viral Compounds;Anti-virals;Intermediates & Fine Chemicals;Non-nucleoside Reverse Transcriptase;Pharmaceuticals
Lopinavir History
In an attempt to improve on the HIV resistance and serum protein-binding properties of the company's earlier protease inhibitor, ritonavir, Lopinavir was developed by Abbott. Abbott pursued a strategy of co-administering lopinavir with sub-therapeutic doses of ritonavir, and lopinavir is only marketed as a co-formulation with ritonavir. It is the first multi-drug capsule to contain a drug not available individually. Lopinavir/ritonavir was approved by the US FDA on 15 September 2000, and in Europe in April 2001. On June 26, 2016, Its patent will expire in the US. Abbott Laboratories was one of the earliest users of the Advanced Photon Source, a national synchrotron-radiation light source at Argonne National Laboratory. One of the early research projects undertaken at the Advanced Photon Source was the Human Immunodeficiency Virus. Using X-ray crystallography, researchers found the points of attack of the HIV protease inhibitors–agents that block the breakdown of proteins. Protease inhibitors stop HIV from making new copies of itself by blocking the last step in the process, when the virus attempts to replicate - and out of that discovery came the drug Kaletra.
Lopinavir Uses
Lopinavir (CAS NO.192725-17-0) is used as a selective HIV protease inhibitor. It is also used as an analogue of Ritonavir.
Lopinavir Specification
The chemical synonyms of Lopinavir (CAS NO.192725-17-0) is also named as (1S-(1R*(R*),3R*,4R*))-N-(4-(((2,6-Dimethylphenoxy)acetyl)amino)-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl)tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide ; (alphaS)-Tetrahydro-N-((alphaS)-alpha-((2S,3S)-2-hydroxy-4-phenyl-3-(2-(2,6-xylyloxy)acetamido)butyl)phenethyl)-alpha-isopropyl-2-oxo-1(2H)-pyrimidineacetamide ; A 157378.0 ; ABT 378 ; ABT-378 ; UNII-2494G1JF75 .
- 192725-45-4(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-(tert-buyloxycarboyl)
- 192725-49-8N-[(1S,2S,4S)-4-amino-2-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]-2-(2,6-dimethylphenoxy)acetamide
- 192725-50-1(2s)-3-Methyl-2-(2-oxotetrahydropyrimidin-1(2h)-yl)butanoic acid
- 192725-55-6(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl 4-nitrophenyl carbonate
- 1927-25-9Homoserine
- 192726-05-9(S)-N-[(2S,4S,5S)-5-Amino-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamide
- 192726-06-0N-(4-Amino-1-benzyl-3-hydroxy-5-phenyl-pentyl)-3-methyl-2-(2-oxo-tetrahydro-pyrimidin-1-yl)-butyramide 5-oxopyrrolidine-2-carboxylic acid
- 1927-31-7Adenosine5'-(tetrahydrogen triphosphate), 2'-deoxy-
- 19275-44-62H,12H-Pyrano[2,3-a]xanthen-12-one,3,4-dihydro-5,9-dihydroxy-10-methoxy-2,2-dimethyl-11-(3-methyl-2-buten-1-yl)-
- 19275-46-82H,6H-Pyrano[3,2-b]xanthen-6-one,3,4-dihydro-5,9-dihydroxy-8-methoxy-2,2-dimethyl-7-(3-methyl-2-butenyl)-
Hot Products
- 75-98-9Pivalic acid
- 5173-46-6Estra-4,9-diene-3,17-dione
- 2386-87-03,4-Epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate
- 557-04-0Octadecanoicacid, magnesium salt (2:1)
- 5451-40-12,6-Dichloropurine
- 143612-79-71H-Indole-5-carbonitrile,3-(4-chlorobutyl)-
- 79-57-2Oxytetracycline
- 109-02-44-Methylmorpholine
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

