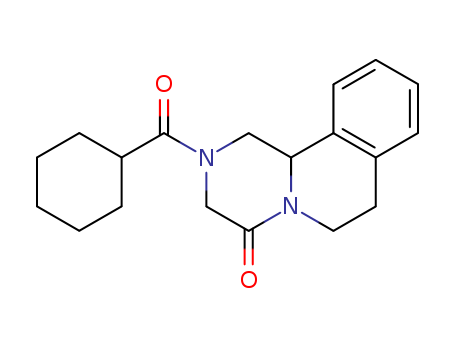
- Chemical Name:Praziquantel
- CAS No.:55268-74-1
- Deprecated CAS:135526-78-2
- Molecular Formula:C19H24N2O2
- Molecular Weight:312.412
- Hs Code.:29339900
- European Community (EC) Number:259-559-6,260-741-2
- NSC Number:757285
- UNII:6490C9U457
- DSSTox Substance ID:DTXSID9021182
- Nikkaji Number:J457.026J,J2.787A
- Wikipedia:Praziquantel
- Wikidata:Q424145
- NCI Thesaurus Code:C47683
- RXCUI:8628
- Metabolomics Workbench ID:43296
- ChEMBL ID:CHEMBL976
- Mol file:55268-74-1.mol
Synonyms:Biltricide;Cesol;Cisticid;Cysticide;Droncit;Drontsit;EMBAY 8440;Prasiquantel;Praziquantel;Praziquantel, (+-)-Isomer;Praziquantel, (R)-Isomer;Praziquantel, (S)-Isomer;Pyquiton;Traziquantel


 F,
F, C
C


