Products Categories
| CAS No.: | 55268-74-1 |
|---|---|
| Name: | Praziquantel |
| Article Data: | 34 |
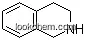
| Molecular Structure: | |
|
|
|
| Formula: | C19H24N2O2 |
| Molecular Weight: | 312.412 |
| Synonyms: | Drontal;Prestwick_402;4H-Pyrazino(2,1-a)isoquinolin-4-one, 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-;Biltricide (TN);2-(Cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino(2,1-a)isoquinolin-4-one;5-24-03-00361 (Beilstein Handbook Reference);2-(Cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro- 4H-pyrazino[2,1-a]-isoquinolin-4-one;Drontal Plus;Droncit;Praziquantel (JAN/USP);Cutter; |
| EINECS: | 259-559-6 |
| Density: | 1.22 g/cm3 |
| Melting Point: | 136-138 °C |
| Boiling Point: | 544.1 °C at 760 mmHg |
| Flash Point: | 254.6 °C |
| Solubility: | Freely soluble in ethanol or dichloromethane. Slightly soluble in water |
| Appearance: | White or practically white crystalline powder |
| Hazard Symbols: |
 F, F, C C
|
| Risk Codes: | 11-34 |
| Safety: | 16-26-36/37/39-45 |
| PSA: | 40.62000 |
| LogP: | 2.41070 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 87693-75-2
4-(cyclohexanecarbonyl)-6-hydroxy-1-phenethylpiperazin-2-one

- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 0℃; | 95% |
| With sulfuric acid | 3.0 g (95%) |
| With sulfuric acid In dichloromethane at 0 - 5℃; for 4h; | 170.1 g |
- 87693-75-2
4-(cyclohexanecarbonyl)-6-hydroxy-1-phenethylpiperazin-2-one


B
- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| In 12N-hydrochloric acid | A 95% B n/a |

- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol at 20℃; under 760.051 Torr; for 19h; Solvent; Inert atmosphere; | 93% |
- 2719-27-9
cyclohexanylcarbonyl chloride

- 61196-37-0
2,3,6,7-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4(11bH)-one

- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| With triethylamine In 1,2-dimethoxyethane for 15h; Ambient temperature; | 90% |
| With sodium carbonate In dichloromethane for 2h; Ambient temperature; | 85% |
| With triethylamine In 1,2-dimethoxyethane for 20h; Ambient temperature; | 70% |
- 406954-89-0
1-[2-(2-bromophenyl)ethyl]-4-(cyclohexylcarbonyl)-1,3,4-trihydropyrazin-2-one

- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In toluene for 16h; Heating; | 90% |
| Multi-step reaction with 2 steps 1: caesium carbonate; tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 18 h / 80 °C / Inert atmosphere 2: palladium on activated charcoal; hydrogen / methanol / 19 h / 20 °C / 760.05 Torr / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: caesium carbonate; tetrakis(triphenylphosphine) palladium(0) / 2-methyltetrahydrofuran / 22 h / 80 °C / Inert atmosphere 2: palladium on activated charcoal; hydrogen / methanol / 19 h / 20 °C / 760.05 Torr / Inert atmosphere View Scheme |
- 79848-93-4
N-(1,2,3,4-tetrahydroisoquinolin-1-ylmethyl)cyclohexanecarboxylic acid amide

- 79-04-9
chloroacetyl chloride

- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane at 20 - 25℃; for 0.7h; | 88% |
| Stage #1: N-(1,2,3,4-tetrahydroisoquinolin-1-ylmethyl)cyclohexanecarboxylic acid amide; chloroacetyl chloride With sodium hydroxide In dichloromethane for 0.5h; Stage #2: With N-benzyl-N,N,N-triethylammonium chloride In dichloromethane for 2h; Reflux; | 81% |
| Stage #1: N-(1,2,3,4-tetrahydroisoquinolin-1-ylmethyl)cyclohexanecarboxylic acid amide; chloroacetyl chloride With sodium hydroxide In dichloromethane at 20℃; for 0.5h; Stage #2: With benzyltrimethylammonium chloride In dichloromethane for 2h; Reflux; | 78% |
| Stage #1: N-(1,2,3,4-tetrahydroisoquinolin-1-ylmethyl)cyclohexanecarboxylic acid amide; chloroacetyl chloride With sodium hydroxide In dichloromethane at 0℃; for 0.5h; Stage #2: With tetrabutylammomium bromide In dichloromethane for 3h; Reflux; | 68% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water Inert atmosphere; | 56% |

- 2719-27-9
cyclohexanylcarbonyl chloride

- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: C14H22N2O3*0.5H2O4S at 35 - 45℃; for 10h; Stage #2: cyclohexanylcarbonyl chloride With lithium hydroxide In water at 5℃; for 5h; | 86.5% |
- 60567-53-5, 60567-54-6, 104916-35-0
1-Cyclohexylcarbonylaminomethyl-2-chloracetyl-1,2,3,4-tetrahydro-isochinolin

- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In benzene at 80℃; for 0.7h; | 86% |

- 2719-27-9
cyclohexanylcarbonyl chloride

- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: C14H22N2O3*H3O4P With phosphoric acid at 10 - 20℃; for 12h; Stage #2: cyclohexanylcarbonyl chloride With calcium bicarbonate In water at 0℃; for 5h; | 86% |
- 2719-27-9
cyclohexanylcarbonyl chloride

- 90142-14-6
N-2-phenylethyl 2-N-(2,2-dimethoxyethylamino)acetamide hydrochloride

- 55268-74-1
2-cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one

| Conditions | Yield |
|---|---|
| Stage #1: N-2-phenylethyl 2-N-(2,2-dimethoxyethylamino)acetamide hydrochloride With sulfuric acid In dichloromethane at 5 - 20℃; for 3.5h; Cooling with ice; Industrial scale; Stage #2: cyclohexanylcarbonyl chloride With sodium carbonate at 5 - 20℃; for 2.5h; Reagent/catalyst; Solvent; Temperature; Industrial scale; | 85.1% |
- 135046-48-9(+/-)-Clopidogrel bisulfate
- 36322-90-4Piroxicam
- 665-66-71-Adamantanamine hydrochloride
- 191732-72-6Lenalidomide
- 288-32-41H-Imidazole
- 209995-38-02,4,5-Trifluorophenylacetic acid
- 151213-42-21H-Pyrrolo[3,4-b]pyridine,octahydro-, (4aR,7aR)-
- 63-91-2L-Phenylalanine
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Chemistry
Molecular Structure:
.png)
Molecular Formula: C19H24N2O2
Molecular Weight: 312.4061
IUPAC Name: 2-(Cyclohexanecarbonyl)-3,6,7,11b-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4-one
Synonyms of Biltricide (CAS NO.55268-74-1): Drontal ; Drontal Plus ; Vercom ; 2-(Cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino(2,1-a)isoquinolin-4-one ; 4H-Pyrazino(2,1-a)isoquinolin-4-one, 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro- ; 5-24-03-00361 (Beilstein Handbook Reference) ; BRN 0761557 ; CCRIS 4114 ; Cutter Tape Tabs ; Droncit ; EINECS 259-559-6 ; Embay 8440 ; Praziquantel ; Praziquantelum ; Praziquantelum [INN-Latin] ; Pyquiton ; UNII-6490C9U457
CAS NO: 55268-74-1
Classification Code: Anthelmintic [veterinary] ; Anthelmintics ; Anti-Infective Agents ; Antiparasitic Agents ; Drug / Therapeutic Agent ; Mutation data
Melting point: 136-138 °C
Index of Refraction: 1.615
Molar Refractivity: 88.76 cm3
Molar Volume: 254.2 cm3
Surface Tension: 55.2 dyne/cm
Density: 1.22 g/cm3
Flash Point: 254.6 °C
Enthalpy of Vaporization: 82.27 kJ/mol
Boiling Point: 544.1 °C at 760 mmHg
Vapour Pressure: 6.71E-12 mmHg at 25°C
History
Laboratories developed the Biltricide for parasitological research of Bayer AG in Germany (Elberfeld) in the mid 1970s. The World Health Organization includes it on its Model List of Essential Medicines.
Uses
1. Biltricide (CAS NO.55268-74-1) is used to treat Echinococcosis
2.It is used to Cysticercosis (though it has been judged less effective than albendazole in treatment of neurocysticercosis.)
3.It is used to Intestinal tapeworms. In veterinary medicine it is widely used against tapeworms, either alone under the trade name Droncit, or in combination with pyrantel pamoate under the trade name Drontal.
4.It is used against Schistosoma.As of 2005, Biltricide is the primary treatment for human schistosomiasis, for which it is usually effective in a single dose.
5.It is used to treat liver flukes (such as Clonorchis sinensis) except for fascioliasis.
6.It is also used to treat paragonimiasis.
Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | oral | > 200mg/kg (200mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 555, 1981. | |
| mouse | LD50 | intramuscular | > 2gm/kg (2000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 555, 1981. | |
| mouse | LD50 | intraperitoneal | 376mg/kg (376mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 20, Pg. 228, 1989. | |
| mouse | LD50 | oral | 2454mg/kg (2454mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 555, 1981. | |
| mouse | LD50 | subcutaneous | 7172mg/kg (7172mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 555, 1981. | |
| rabbit | LD50 | oral | 1050mg/kg (1050mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 555, 1981. | |
| rat | LD50 | intramuscular | > 2gm/kg (2000mg/kg) | Drugs in Japan Vol. -, Pg. 965, 1990. | |
| rat | LD50 | intraperitoneal | 586mg/kg (586mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 20, Pg. 228, 1989. | |
| rat | LD50 | oral | 2840mg/kg (2840mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 555, 1981. | |
| rat | LD50 | subcutaneous | > 16gm/kg (16000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 555, 1981. |
Consensus Reports
EPA Genetic Toxicology Program.
Safety Profile
Hazard Codes of Biltricide (CAS NO.55268-74-1):  F,
F, C
C
Risk Statements: 11-34
R11: Highly flammable.
R34: Causes burns.
Safety Statements: 16-26-36/37/39-45
S16: Keep away from sources of ignition.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
WGK Germany: 1
RTECS: UQ4150000
Poison by intraperitoneal route. Moderately toxic by ingestion and other routes. Human mutation data reported. When Biltricide was heated to decomposition, it emits toxic fumes of NOx.