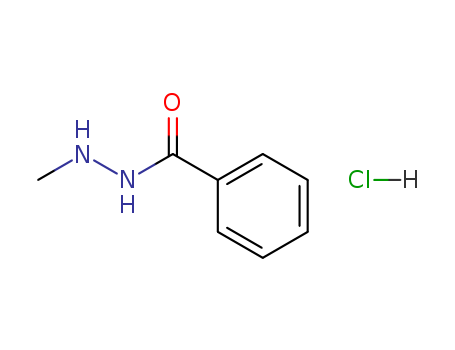
- Chemical Name:N'-Methylbenzohydrazide--hydrogen chloride (1/1)
- CAS No.:1483-23-4
- Molecular Formula:C8H10 N2 O . Cl H
- Molecular Weight:150.1778
- Hs Code.:
- DSSTox Substance ID:DTXSID10494956
- NSC Number:79065
- Mol file:1483-23-4.mol
Synonyms:1483-23-4;N'-Methylbenzohydrazide--hydrogen chloride (1/1);DTXSID10494956;NSC79065;NSC-79065