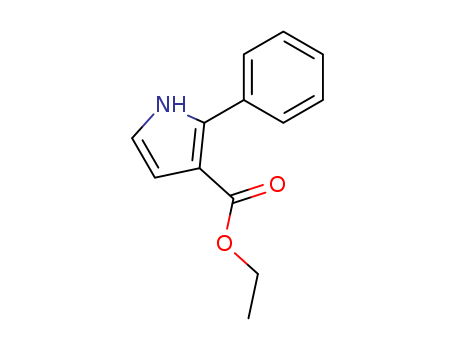
- Chemical Name:ethyl 2-phenyl-1H-pyrrole-3-carboxylate
- CAS No.:38597-58-9
- Molecular Formula:C13H13NO2
- Molecular Weight:215.252
- Hs Code.:
- Mol file:38597-58-9.mol
Synonyms:1H-Pyrrole-3-carboxylic acid, 2-phenyl-, ethyl ester;2-Phenyl-1H-pyrrole-3-carboxylic acid ethyl ester;