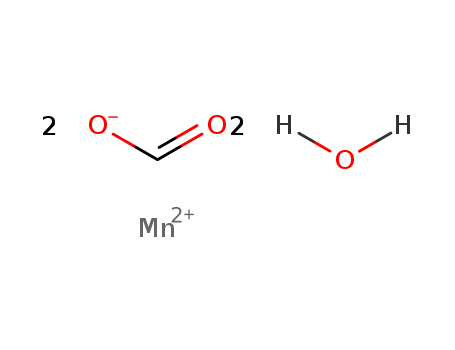
- Chemical Name:MANGANESE(II) DIFORMATE DIHYDRATE
- CAS No.:4247-36-3
- Molecular Formula:2CHO2*2H2O*Mn
- Molecular Weight:181.004
- Hs Code.:
- Mol file:4247-36-3.mol
Synonyms:Formicacid,manganese(2+) salt (8CI,9CI);Manganese(II) formate;Manganesediformate;Manganese formate (6CI,7CI);Ameisensaeure,Mangan(II)-formiat;manganese(II) bis(formira) dihydrate;Formic acid,manganese(2+) salt (2:1);MANGANESE FORMATE;Manganese(2+) formate;Manganous formate;manganese formate dihydrate;manganese(II) formate dihydrate;formic acid,manganese (II)-formate;Mn(II)-formate *2H2O;Mn dihydrate formate;