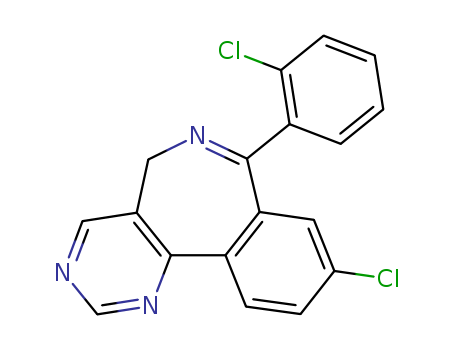
- Chemical Name:9-chloro-7-(2-chlorophenyl)-5H-pyrimido(5,4-d)(2)benzazepine
- CAS No.:76988-39-1
- Molecular Formula:C18H11 Cl2 N3
- Molecular Weight:340.211
- Hs Code.:2933990090
- Mol file:76988-39-1.mol
Synonyms:9-Chloro-7-(2-chlorophenyl)-5H-pyrimido[5,4-d][2]benzazepine;Ro 22-3245