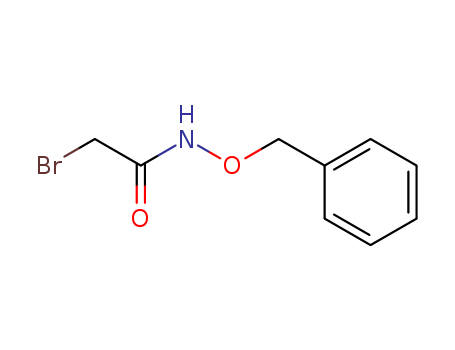
- Chemical Name:N-(Benzyloxy)-2-bromoacetamide
- CAS No.:78158-32-4
- Molecular Formula:C9H10BrNO2
- Molecular Weight:244.088
- Hs Code.:
- DSSTox Substance ID:DTXSID10572748
- Nikkaji Number:J443.954F
- Wikidata:Q82461225
- Mol file:78158-32-4.mol
Synonyms:N-(Benzyloxy)-2-bromoacetamide;78158-32-4;Bromo-N-(benzyloxyl)acetamide;N-Benzyloxy-2-bromo-acetamide;SCHEMBL5557749;DTXSID10572748;JJEUVHILRLHHEM-UHFFFAOYSA-N;AKOS014304327