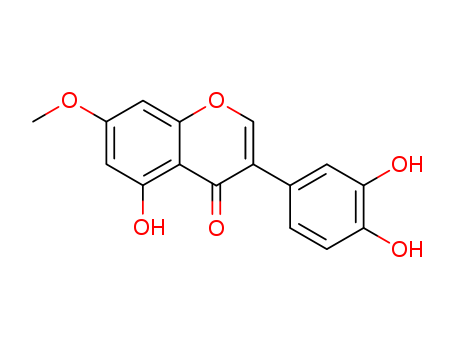
- Chemical Name:Santal
- CAS No.:529-60-2
- Molecular Formula:C16H12O6
- Molecular Weight:300.268
- Hs Code.:
- Nikkaji Number:J11.879F
- Wikidata:Q104402253
- Metabolomics Workbench ID:22483
- Mol file:529-60-2.mol
Synonyms:Santal;529-60-2;5,3',4'-Trihydroxy-7-methoxyisoflavone;3-(3,4-dihydroxyphenyl)-5-hydroxy-7-methoxychromen-4-one;SCHEMBL2313317;CHEBI:174860;LMPK12050346;3',4',5-trihydroxy-7-methoxyisoflavone