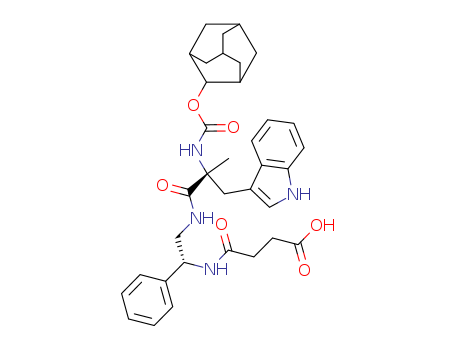
- Chemical Name:4-[[(1R)-2-[[(2R)-2-(2-adamantyloxycarbonylamino)-3-(1H-indol-3-yl)-2-methylpropanoyl]amino]-1-phenylethyl]amino]-4-oxobutanoic acid
- CAS No.:130332-27-3
- Molecular Formula:C35H42N4O6
- Molecular Weight:614.742
- Hs Code.:
- UNII:2637PDX9SI
- DSSTox Substance ID:DTXSID701099873
- Nikkaji Number:J360.238I
- Wikipedia:CI-988
- Wikidata:Q76009969
- Pharos Ligand ID:UB3APZZPPH7V,UB3XXNJFQJ9J
- ChEMBL ID:CHEMBL2062154,CHEMBL287735
- Mol file:130332-27-3.mol
Synonyms:4-((2-((3-(1H-indol-3-yl)-2-methyl-1-oxo-2-(((tricyclo(3.3.1.1)-dec-2-yloxy)carbonyl)amino)propyl)amino)-1-phenethyl)amino)-4-oxobutanoate N-methyl-D-glucamine;CI 988;CI-988;PD 134308;PD-134308;PD134308