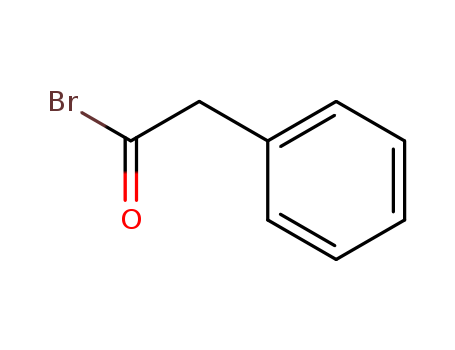
- Chemical Name:Phenylacetyl bromide
- CAS No.:22535-03-1
- Molecular Formula:C8H7 Br O
- Molecular Weight:199.047
- Hs Code.:2916399090
- European Community (EC) Number:245-060-0
- DSSTox Substance ID:DTXSID30177052
- Nikkaji Number:J286.236K
- Wikidata:Q83047373
- Mol file:22535-03-1.mol
Synonyms:Phenylacetyl bromide;2-phenylacetyl bromide;22535-03-1;EINECS 245-060-0;phenacetyl bromide;phenylacetylbromide;Benzeneacetic acid bromide;SCHEMBL2186535;DTXSID30177052;AKOS006272488