10.1016/j.ejmech.2010.03.008
The research study on the synthesis and structure-activity relationship of 8-hydroxyquinoline-derived Mannich bases as potential anticancer agents. The purpose of the study was to explore the growth-inhibitory effects of these compounds on various human carcinoma cell lines and to understand the impact of structural modifications on their potency. The researchers synthesized a series of Mannich bases and assessed their activity against cell lines including HeLa, BT483, SKHep, and CE81T using the MTT assay. The conclusions drawn from the study emphasized that the 8-hydroxyquinoline scaffold is crucial for activity, and certain structural modifications, such as the introduction of flexible fragments and specific substituents, significantly enhanced the growth-inhibitory effects. Notably, compound 25 emerged as the most active against HeLa and BT483 cells, while compounds 19 and 26 showed potent effects on SKHep and CE81T cells, respectively. The study utilized a variety of chemicals in the synthesis process, including 8-hydroxyquinoline, phenol, 3-hydroxypyridine, 1-naphthol, and various substituted phenylsulfonyl and piperazine derivatives. The 3D-QSAR analysis further revealed that both steric and electronic effects contributed equally to the growth inhibition, providing valuable insights for future structural optimizations.
10.1021/jo902226t
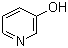
The study focuses on the synthesis and evaluation of 3-pyridinols, which are compounds carrying alkyltelluro, alkylseleno, and alkylthio groups, as potent antioxidant agents. These pyridinols were tested for their ability to inhibit azo-initiated peroxidation of linoleic acid in a water/chlorobenzene two-phase system and in a homogeneous phase. The chemicals used in the study include various 3-pyridinols with different substituents, N-acetylcysteine (NAC) as a water-soluble co-antioxidant, and N-tert-butoxycarbonyl cysteine methyl ester (LipCys), a lipid-soluble analogue of NAC. The purpose of these chemicals was to assess their antioxidant activity, specifically their capacity to quench peroxyl radicals, regenerate through reduction by thiol reducing agents, and their potential catalytic antioxidant behavior. The study aimed to understand the kinetic, thermodynamic, and mechanistic aspects of these antioxidants' activities and to identify the structural features that contribute to their high reactivity and effectiveness.


 Xi,
Xi, Xn
Xn

