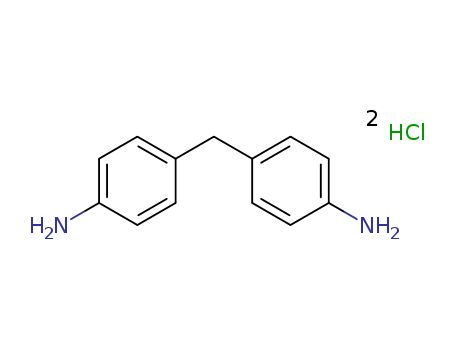
- Chemical Name:4,4'-Methylenedianiline
- CAS No.:13552-44-8
- Deprecated CAS:28602-61-1,83712-44-1,120859-32-7,136601-30-4,148263-71-2,120859-32-7,148263-71-2,83712-44-1
- Molecular Formula:C13H14 N2 . 2 Cl H
- Molecular Weight:271.189
- Hs Code.:
- European Community (EC) Number:202-974-4,236-934-2,663-410-4
- ICSC Number:1111
- NSC Number:4709
- UN Number:2651
- UNII:GG5LL7OBZC
- DSSTox Substance ID:DTXSID6022422
- Nikkaji Number:J4.995F
- Wikipedia:4,4%27-Methylenedianiline,4'-Methylenedianiline
- Wikidata:Q229848
- NCI Thesaurus Code:C44320
- Metabolomics Workbench ID:49551
- ChEMBL ID:CHEMBL85728
- Mol file:13552-44-8.mol
Synonyms:4,4'-diaminodiphenylmethane;4,4'-diaminodiphenylmethane dihydrochloride;4,4'-diaminodiphenylmethane, sodium chloride (3:1);4,4'-MDA;4,4'-methylene dianiline;4,4'-methylenebisaniline;4,4'-methylenedianiline;di(4-aminophenyl)methane;p,p'-diaminodiphenylmethane;Tonox