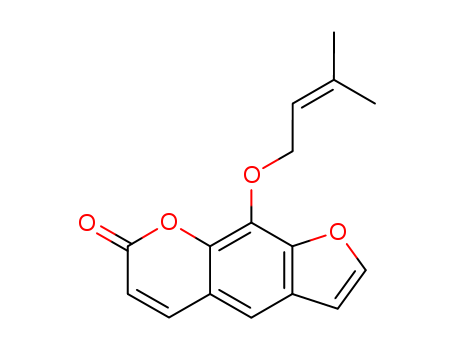
- Chemical Name:Imperatorin
- CAS No.:482-44-0
- Deprecated CAS:70102-00-0
- Molecular Formula:C16H14O4
- Molecular Weight:270.285
- Hs Code.:29419090
- European Community (EC) Number:207-581-1
- NSC Number:402949
- UNII:K713N25C78
- DSSTox Substance ID:DTXSID8048737
- Nikkaji Number:J25.135F
- Wikipedia:Imperatorin
- Wikidata:Q1649534
- NCI Thesaurus Code:C63672
- Pharos Ligand ID:PSM3XMRCLX2A
- Metabolomics Workbench ID:46512
- ChEMBL ID:CHEMBL453805
- Mol file:482-44-0.mol
Synonyms:5-hydroxy-8-(1,1-dimethylallyl)psoralen;8-isoamylenoxypsoralen;imperatorin