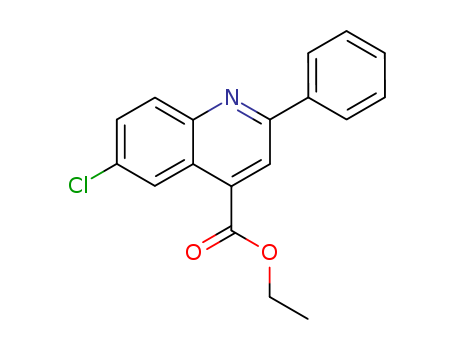
- Chemical Name:Ethyl 6-chloro-2-phenylquinoline-4-carboxylate
- CAS No.:6633-64-3
- Molecular Formula:C18H14 Cl N O2
- Molecular Weight:311.76
- Hs Code.:2933499090
- NSC Number:42127
- DSSTox Substance ID:DTXSID20984934
- Wikidata:Q82972319
- Mol file:6633-64-3.mol
Synonyms:ethyl 6-chloro-2-phenylquinoline-4-carboxylate;NSC42127;SCHEMBL11173303;DTXSID20984934;NSC-42127