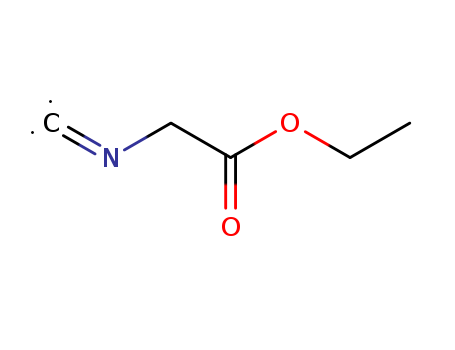
- Chemical Name:Ethyl isocyanoacetate
- CAS No.:2999-46-4
- Molecular Formula:C5H7NO2
- Molecular Weight:113.116
- Hs Code.:29299090
- European Community (EC) Number:221-077-9
- DSSTox Substance ID:DTXSID20184078
- Nikkaji Number:J150.099F
- Wikidata:Q72475591
- Mol file:2999-46-4.mol
Synonyms:Ethyl isocyanoacetate;2999-46-4;ethyl 2-isocyanoacetate;Isocyanoacetic Acid Ethyl Ester;Acetic acid, isocyano-, ethyl ester;EINECS 221-077-9;ETHYLISOCYANOACETATE;MFCD00000007;Acetic acid, 2-isocyano-, ethyl ester;ethyl iso-cyanoacetate;ethyl isocyano-acetate;ethyl 2-isocyanoacetat;ethyl isocyano- acetate;ethyl-2-isocyanoacetate;SCHEMBL7225;Ethyl isocyanoacetate(EICA);isocyano-acetic acid ethylester;AMY403;isocyano-acetic acid ethyl ester;DTXSID20184078;BCP11231;STR02697;BBL021319;GEO-01356;STK894028;AKOS005144095;AB00104;SB40700;2-ISOCYANO-ACETIC ACID ETHYL ESTER;BB 0262985;FT-0627402;I0562;EN300-50660;J-017729;J-521262;F0001-2483


 Xn
Xn

