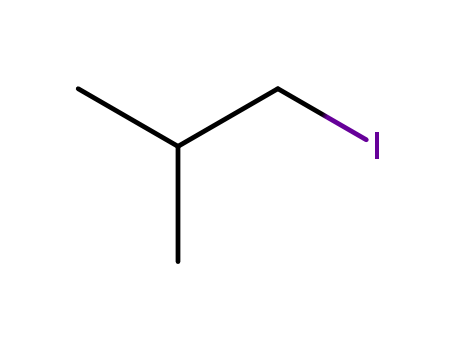
- Chemical Name:1-Iodo-2-methylpropane
- CAS No.:513-38-2
- Molecular Formula:C4H9I
- Molecular Weight:184.02
- Hs Code.:29033080
- European Community (EC) Number:208-160-5
- NSC Number:8421
- UN Number:2391
- UNII:82IJ6B9708
- DSSTox Substance ID:DTXSID9060154
- Nikkaji Number:J2.620D
- Wikidata:Q27269335
- Mol file:513-38-2.mol
Synonyms:Isobutyl iodide;1-IODO-2-METHYLPROPANE;513-38-2;Propane, 1-iodo-2-methyl-;Isobutyljodid;2-Methylpropyl iodide;Primary isobutyl iodide;Isobutyljodid [Czech];1-Jod-2-methylpropan;1-iodo-2-methyl-propane;1-Jod-2-methylpropan [Czech];NSC 8421;EINECS 208-160-5;BRN 1730927;UNII-82IJ6B9708;NSC-8421;82IJ6B9708;isobutyliodide;iodoisobutane;isobutyl iodine;1-iodoisobutane;iso-butyl iodide;2-methylpropyliodide;2-methyl-iodopropane;MFCD00001084;Iodo-2-methylpropane;iso-C4H9I;3-iodo-2-methylpropane;1-iodo-2-methyl propane;1-iodanyl-2-methyl-propane;C4H9I;SCHEMBL28155;ISOBUTYL IODIDE [MI];DTXSID9060154;WLN: I1Y1&1;NSC8421;STR01905;NA2391;STL141079;UN2391;AKOS000119847;1-Iodo-2-methylpropane stabilized over Cu;LS-120830;FT-0607949;I0322;EN300-19804;H11266;A828543;1-IODO-2-METHYLPROPANE (STAB. WITH COPPER);J-802035;Q27269335;F2190-0185;1-Iodo-2-methylpropane, contains copper as stabilizer, 97%


 F,
F, Xi,
Xi, Xn
Xn


