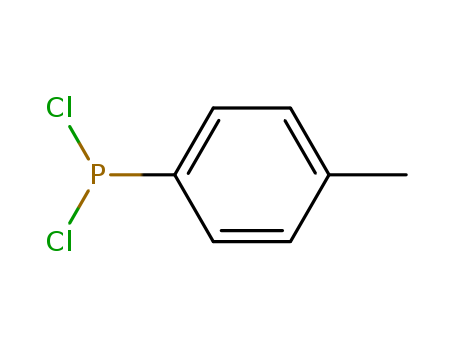
- Chemical Name:Phosphonous dichloride, (4-methylphenyl)-
- CAS No.:1005-32-9
- Molecular Formula:C7H7 Cl2 P
- Molecular Weight:193.012
- Hs Code.:
- DSSTox Substance ID:DTXSID1074335
- Nikkaji Number:J1.455.080A
- Wikidata:Q82002710
- Mol file:1005-32-9.mol
Synonyms:1005-32-9;Phosphonous dichloride, (4-methylphenyl)-;Phosphonous dichloride, P-(4-methylphenyl)-;p-Tolyldichlorophosphine;SCHEMBL2367541;DTXSID1074335