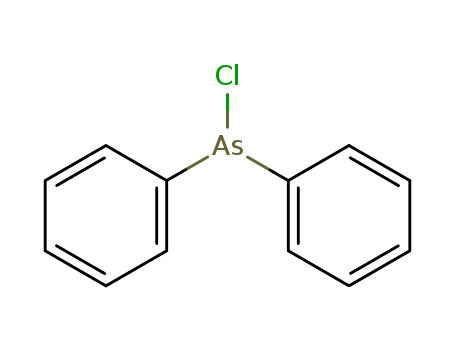
- Chemical Name:Diphenylchloroarsine
- CAS No.:712-48-1
- Molecular Formula:C12H10 As Cl
- Molecular Weight:264.586
- Hs Code.:2931900036
- European Community (EC) Number:211-921-4
- ICSC Number:1526
- UN Number:1699
- UNII:1H39V3559B
- DSSTox Substance ID:DTXSID30858733
- Nikkaji Number:J35.211J
- Wikipedia:Diphenylchlorarsine
- Wikidata:Q421470
- Mol file:712-48-1.mol
Synonyms:Clark 1;diphenyl arsine chloride