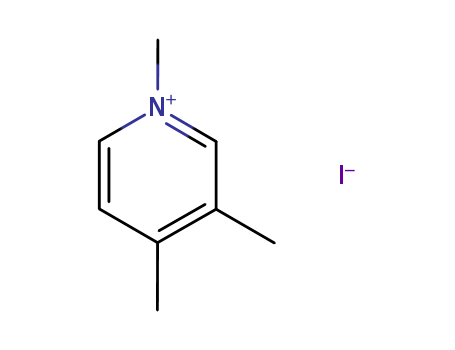
- Chemical Name:1,3,4-Trimethylpyridinium iodide
- CAS No.:6283-41-6
- Molecular Formula:C8H12N+
- Molecular Weight:249.094
- Hs Code.:
- DSSTox Substance ID:DTXSID40501945
- NSC Number:7493
- Mol file:6283-41-6.mol
Synonyms:1,3,4-trimethylpyridinium iodide;6283-41-6;1,3,4-trimethyl-pyridinium iodide;1,3,4-Trimethyl-pyridinium;iodide;SCHEMBL3857270;DTXSID40501945;NSC7493;LNUYBCUFHJCJFD-UHFFFAOYSA-M;NSC-7493;1,3,4-Trimethylpyridin-1-ium iodide;1,3,4-trimethylpyridin-1-ium;AKOS024388294