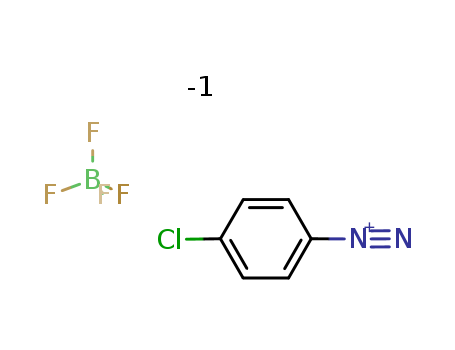10.1021/acs.joc.7b03098
The research explores the development of novel palladium-catalyzed redox-relay Heck arylation reactions. The study focuses on the use of trisubstituted allylic alkenols and their silyl and methyl ether derivatives to form α,β-disubstituted methyl ketones containing two contiguous stereocenters. Key chemicals involved in the research include various trisubstituted alkenols (such as (E)-3-methylhex-3-en-2-ol), silyl ethers (like tert-butyldimethyl((3-methylhex-3-en-2-yl)oxy)silane), and methyl ethers (such as (E)-2-methoxy-3-methylhex-3-ene). Arenediazonium salts, such as 4-chlorobenzenediazonium tetrafluoroborate, also play a crucial role as electrophiles in the Heck-Matsuda reaction. The reactions are catalyzed by palladium complexes like Pd(TFA)2 and proceed under mild conditions in methanol, yielding products with excellent anti diastereoselectivity. The study demonstrates that the presence of a free hydroxyl group is not essential for an effective redox-relay process, as silyl and methyl ethers can also undergo late oxidation. This method was successfully applied in the total synthesis of meso-hexestrol, showcasing its potential for constructing complex organic structures.