10.1002/anie.201001242
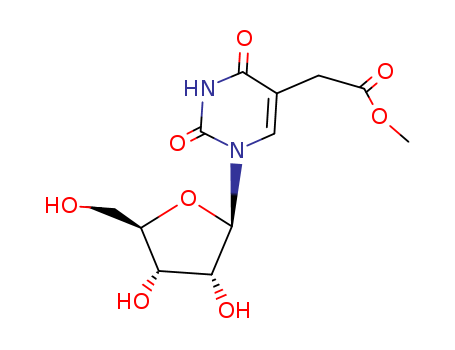
The research focuses on the AlkB domain of the mammalian ABH8 protein, which is part of the AlkB family of nonheme iron/α-ketoglutarate (aKG)-dependent dioxygenases. The study aimed to investigate the biochemical activity of ABH8, particularly its role in RNA modification. The researchers hypothesized that ABH8 could modify tRNAs through a controlled methylation/demethylation process. Through a series of experiments, they discovered that the AlkB domain of ABH8 catalyzes the hydroxylation of 5-methoxycarbonylmethyluridine (mcm5U) at the wobble position of tRNA, converting it into 5-(S)-methoxycarbonylhydroxymethyluridine ((S)-mchm5U). This modification was found to be specific to ABH8 and required the presence of iron(II) and aKG cofactors. The chemicals used in the process included iron and aKG as cofactors, l-ascorbic acid, and EDTA for quenching the reaction. The conclusions of the study suggest that ABH8 may play a role in regulating RNA function and could have implications in cancer progression, as it was previously shown to affect cancer cells. The findings also expand the understanding of the AlkB family of proteins, which were previously known for their demethylation activities, to include modification functions on nucleic acids.