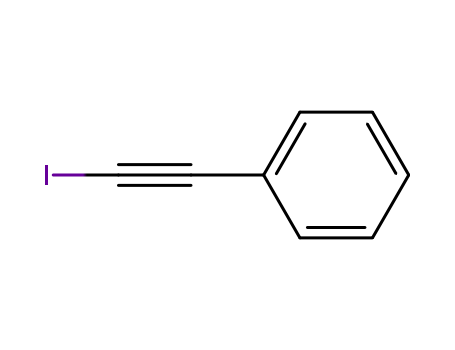10.1021/ol800105r
The research focuses on the indium-catalyzed syn-addition of 1,3-dicarbonyl compounds to 1-iodoalkynes, resulting in the stereoselective synthesis of E-iodoalkenes. The iodine atom plays a dual role as both an activating group and a regioselectivity controller. The experiments involved reacting 1,3-dicarbonyl compounds with 1-iodo-2-phenylacetylenes in the presence of 5 mol % of In(NTf2)3 catalyst in toluene at 50 °C for 12 hours. The reactions were found to be entirely regioselective and E-stereoselective, yielding a single isomer with high yields. The products were characterized using X-ray crystallographic analysis, confirming the E-configuration. The scope of the reaction was further explored with various 1,3-dicarbonyl compounds and iodoalkynes, with the results indicating that electron-rich iodoalkynes are reactive under the reaction conditions, while electron-deficient ones require higher temperatures and longer times. The addition products were then transformed into trisubstituted olefins using cross-coupling reactions such as Sonogashira and Suzuki couplings. The reaction mechanism and the synthetic utility of the products were highlighted as intriguing aspects of the study.
10.1055/s-1998-6087
The research investigates the copper-catalyzed cross-coupling of 1-iodoalkynes with organostannanes to synthesize enynes, aiming to develop an efficient and practical method for this transformation. The study finds that using CuI as the catalyst in DMF at room temperature, and slowly adding 1-iodoalkynes to organostannanes via a syringe pump over 6 hours, significantly reduces homocoupling and increases the yield of the desired cross-coupled products. Key chemicals used include 1-iodoalkynes such as 1-iodo-2-phenylacetylene and various organostannanes like 2-furylstannane and 2-thienylstannane. The method is shown to be effective with different types of organostannanes, yielding products in good to excellent yields, demonstrating its versatility and potential for synthesizing a range of enynes.