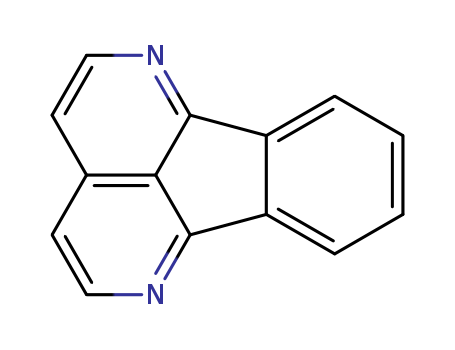
- Chemical Name:Eupolauridine
- CAS No.:58786-39-3
- Molecular Formula:C14H8 N2
- Molecular Weight:204.23
- Hs Code.:
- UNII:M3LXQ9G7MF
- DSSTox Substance ID:DTXSID80974311
- Nikkaji Number:J13.838J
- Wikidata:Q5851990
- Metabolomics Workbench ID:44239
- ChEMBL ID:CHEMBL477708
- Mol file:58786-39-3.mol
Synonyms:eupolauridine;indeno(1,2,3-ij)(2,7)naphthyridine