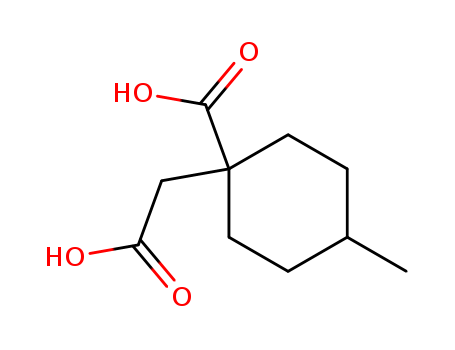
- Chemical Name:1-(Carboxymethyl)-4-methylcyclohexanecarboxylic acid
- CAS No.:5433-06-7
- Molecular Formula:C10H16O4
- Molecular Weight:200.235
- Hs Code.:
- NSC Number:29097
- DSSTox Substance ID:DTXSID90283007
- ChEMBL ID:CHEMBL1895622
- Mol file:5433-06-7.mol
Synonyms:5433-06-7;1-(carboxymethyl)-4-methylcyclohexane-1-carboxylic acid;MLS002639259;54324-42-4;cis-1-(Carboxymethyl)-4-methylcyclohexanecarboxylic acid;1-(carboxymethyl)-4-methylcyclohexanecarboxylic acid;NSC29097;CHEMBL1895622;DTXSID90283007;HMS3080E13;NSC-29097;SMR001548705