- Chemical Name:4-Cyanobenzyl bromide
- CAS No.:17201-43-3
- Molecular Formula:C8H6BrN
- Molecular Weight:196.046
- Hs Code.:29269090
- European Community (EC) Number:241-246-0
- NSC Number:95792
- UNII:RV332B84ZG
- DSSTox Substance ID:DTXSID40864744
- Nikkaji Number:J266.795I
- Wikidata:Q72457942
- Mol file:17201-43-3.mol
Synonyms:4-Cyanobenzyl bromide;17201-43-3;4-(Bromomethyl)benzonitrile;p-Cyanobenzyl bromide;Benzonitrile, 4-(bromomethyl)-;alpha-Bromo-p-tolunitrile;4-bromomethylbenzonitrile;4-Bromomethyl-benzonitrile;alpha-Bromo-p-toluonitrile;p-(Bromomethyl)benzonitrile;Benzonitrile, p-(bromomethyl)-;.alpha.-Bromo-p-tolunitrile;4-cyanobenzylbromide;NSC 95792;p-Tolunitrile, alpha-bromo-;p-Toluonitrile, alpha-bromo-;RV332B84ZG;p-Tolunitrile, .alpha.-bromo-;p-Toluonitrile, .alpha.-bromo-;EINECS 241-246-0;NSC-95792;4-Cyano benzyl bromide;4CNBB;A-BROMO-P-TOLUNITRILE;AKOS BC-3028;1-(bromomethyl)-4-cyanobenzene;p-cyanobenzylbromide;4-cyano benzylbromide;4-cyano-benzylbromide;4-cyanobenzyl-bromide;a-bromo p-tolunitrile;a-bromo-p-toluonitrile;p-cyano-benzyl bromide;4-cyano-benzyl bromide;para-cyanobenzyl bromide;alpha-bromo-4-tolunitrile;4-bromomethyl benzonitrile;alpha-bromo-p-cyanotoluene;SCHEMBL531;alpha-bromo-4-toluonitrile;NCIOpen2_001738;UNII-RV332B84ZG;1-bromomethyl-4-cyanobenzene;4-(bromo-methyl)benzonitrile;4-(bromomethyl)-benzonitrile;(4-cyanophenyl)methyl bromide;4-(bromo-methyl)-benzonitrile;Benzonitrilo, 4-(bromometil)-;DTXSID40864744;4-(Bromomethyl)benzonitrile, 99%;BCP22608;NSC95792;STL168878;AKOS000268730;AC-8256;CS-W001931;GS-3163;BP-21342;AM20060010;BB 0259257;C1398;FT-0622123;EN300-17712;10.14272/UMLFTCYAQPPZER-UHFFFAOYSA-N;A25002;A905245;doi:10.14272/UMLFTCYAQPPZER-UHFFFAOYSA-N.1;Q-200404;Z56995138;4-(Bromomethyl)benzonitrile, purum, >=95.0% (HPLC)

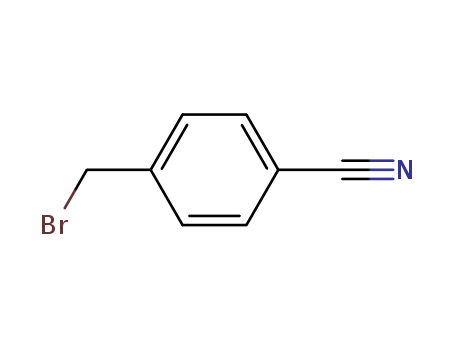

 C
C


