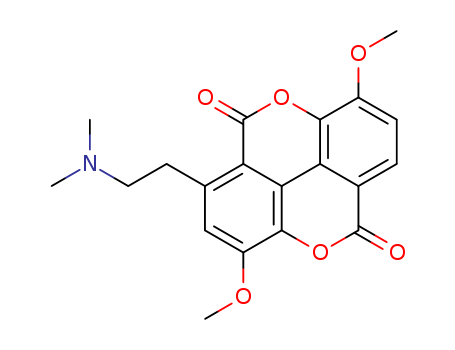
Multi-step reaction with 8 steps
1: 1.) dicyclohexylcarbodiimide, N-hydroxysuccinimide / 1) THF, acetonitrile, rt, 12 h; 2) THF, CH3CM, reflux, 2 h
2: 1.) NaOH, Adogen 464 / 1) CH2Cl2, water, rt, 30 min; 2) CH2Cl2, water, 0 deg C, 1 h
3: 1.) n-BuLi, 2.) I2 / 1) THF, pentane, -60 deg C, 2 h; 2) THF, pentane, -60 deg C --> rt, 1 h
4: 66 percent / copper bronze / dimethylformamide / 3 h / Heating
5: 1.) n-BuLi, 2.) I2 / 1) THF, pentane, -60 deg C, 2 h; 2) THF, pentane, -60 deg C --> rt, 1 h
6: 82 percent / tetrakis(triphenylphosphine)palladium(0) / dioxane / 14 h / Heating
7: 1.) O3, 1.) dimethyl sulfide / 1) 1,2-dichloroethane, -78 deg C; 2) 1,2-dichloroethane, -78 deg C, 1 h, 0 deg C, 1 h, rt, 1 h
8: 75 percent / sodium triacetoxyborohydride / 1,2-dichloro-ethane / 0.17 h / Ambient temperature
With
sodium hydroxide; 1-hydroxy-pyrrolidine-2,5-dione; tetrakis(triphenylphosphine) palladium(0); n-butyllithium; adogen 464; dimethylsulfide; iodine; sodium tris(acetoxy)borohydride; copper; ozone; dicyclohexyl-carbodiimide;
In
1,4-dioxane; 1,2-dichloro-ethane; N,N-dimethyl-formamide;
DOI:10.1021/jo981099j