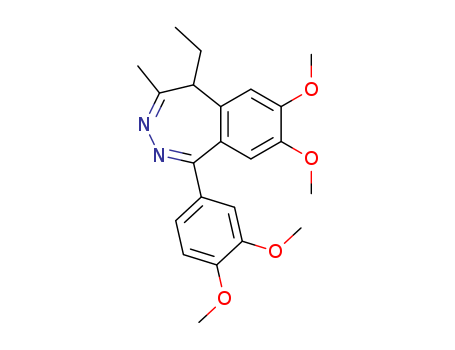
- Chemical Name:Tofisopam
- CAS No.:22345-47-7
- Molecular Formula:C22H26N2O4
- Molecular Weight:382.459
- Hs Code.:
- European Community (EC) Number:244-922-3
- UNII:UZC80HAU42
- DSSTox Substance ID:DTXSID3023681
- Nikkaji Number:J11.504E
- Wikipedia:Tofisopam
- Wikidata:Q945537
- NCI Thesaurus Code:C90791
- Pharos Ligand ID:KZFLKDCNGXJS
- Metabolomics Workbench ID:43726
- ChEMBL ID:CHEMBL404216
- Mol file:22345-47-7.mol
Synonyms:1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5H-2,3-benzodiazepine;dextofisopam;EGYT-341;Grandaxin;levotofisopam;tofisopam;tofizopam


 Xn,
Xn, N
N


