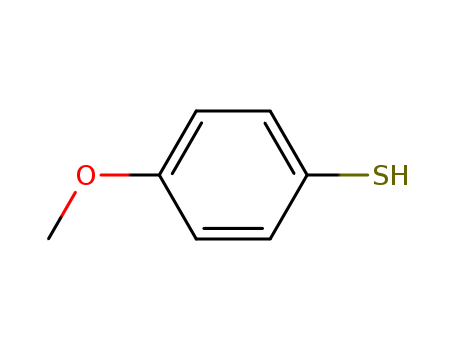
- Chemical Name:4-Methoxybenzenethiol
- CAS No.:696-63-9
- Molecular Formula:C7H8OS
- Molecular Weight:140.206
- Hs Code.:29309090
- European Community (EC) Number:211-799-2
- NSC Number:100727
- UNII:68N68LMU0G
- DSSTox Substance ID:DTXSID60219873
- Nikkaji Number:J54.510D
- Wikidata:Q72486214
- ChEMBL ID:CHEMBL119636
- Mol file:696-63-9.mol
Synonyms:4-Methoxybenzenethiol;4-Methoxythiophenol;696-63-9;Benzenethiol, 4-methoxy-;p-Methoxybenzenethiol;BENZENETHIOL, p-METHOXY-;4-methoxybenzene-1-thiol;4-Methoxy thiophenol;4-Methoxy-benzenethiol;4-Mercaptoanisole;p-methoxythiophenol;NSC 100727;para-Methoxybenzenethiol;EINECS 211-799-2;BRN 1446910;68N68LMU0G;NSC-100727;4-mercaptoanisol;4methoxythiophenol;4methoxybenzenethiol;4-methoxybenzenthiol;4-methoxyphenylthiol;p-Methoxy thiophenol;4-methoxy-thiophenol;NSC100727;4-methoxylbenzenethiol;4-methoxy benzenethiol;4-methoxybenzene thiol;P-MERCAPTOANISOLE;4-methoxy-benzene thiol;4-(methyloxy)benzenethiol;4-methoxy-1-benzenethiol;P-METHOXYPHENYLTHIOL;WLN: SHR DO1;4-Methoxythiophenol, 97%;UNII-68N68LMU0G;SCHEMBL50514;CHEMBL119636;METHOXYBENZENETHIOL, 4-;P-METHOXYPHENYL MERCAPTAN;4-METHOXYPHENYL MERCAPTAN;NIFAOMSJMGEFTQ-UHFFFAOYSA-;DTXSID60219873;CS-D1129;STR00751;BBL027485;MFCD00004849;STL374080;AKOS005203301;AC-7112;LS-32193;FT-0618859;M1333;4-Methoxythiophenol, purum, >=98.0% (GC);EN300-11815;D78094;A836591;W-104608;F0001-1809;QU5


 Xn
Xn

