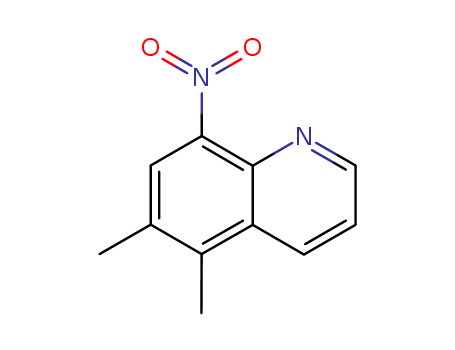
- Chemical Name:5,6-Dimethyl-8-nitroquinoline
- CAS No.:68527-68-4
- Molecular Formula:C11H10N2O2
- Molecular Weight:202.213
- Hs Code.:
- European Community (EC) Number:271-275-4
- UNII:NCW3FX3SKP
- DSSTox Substance ID:DTXSID6071611
- Nikkaji Number:J309.153H
- Wikidata:Q81999322
- Mol file:68527-68-4.mol
Synonyms:5,6-Dimethyl-8-nitroquinoline;68527-68-4;NCW3FX3SKP;Quinoline, 5,6-dimethyl-8-nitro-;EINECS 271-275-4;UNII-NCW3FX3SKP;DTXSID6071611;SCHEMBL11973643;QUINOLINE,5,6-DIMETHYL-8-NITRO-