10.1016/S0040-4039(00)91687-3
The study investigates the reduction of enamides using an NADH model compound. The researchers explored the regioselectivity of this reduction process, finding that the geometry and electronic properties of the substrates significantly influence the reduction outcomes. Cyclic enamides, such as 2a and 2d, were successfully reduced, while non-cyclic derivatives like 2b and 2c were not reduced. The study utilized deuterated models to determine the site of hydrogen attack, revealing that the nitrogen lone pair in the enamide structure governs the regioselectivity. The reduction process was facilitated by magnesium ions, which are believed to activate the substrate and form a ternary complex with the reagent. The study also examined the role of various substituents, such as methoxy groups, in enhancing the reactivity of the substrates. The findings provide insights into the mechanisms of enamide reduction and highlight the importance of substrate geometry and electronic factors in determining the efficiency of the reduction process.


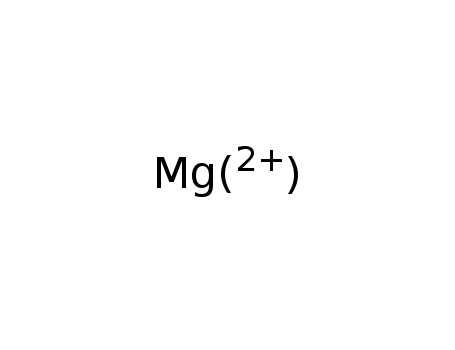
 Xi
Xi


