10.1016/S0040-4020(02)00526-4
The research focuses on the synthesis and application of a new soluble polymer analogue of Wang resin in solid-phase chemistry, specifically for inter-molecular radical additions of xanthates onto olefins. The experiments compared the new resin with a classical Wang resin, examining the efficiency of radical transfer and cleavage conditions. Reactants included xanthates, olefins, and the new soluble polymer, with reactions monitored using 'H NMR spectroscopy for easy tracking of the reaction progress. The analyses involved various techniques such as 'H and "C NMR, infrared spectroscopy, mass spectrometry, HPLC, and TLC to characterize the reactants, intermediates, and final products. The study demonstrated that the new soluble polymer provided better results for radical transfer, preserved the efficient cleavage conditions of the Wang linker, and allowed for easier monitoring of reactions compared to the classical Wang resin.
10.1016/0040-4039(96)01253-1
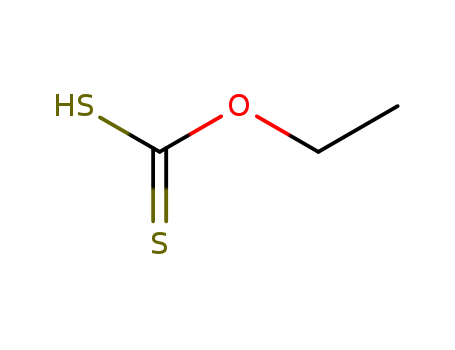
The research describes a practical method for the reductive cleavage of the sulfide bond in xanthates, with the purpose of removing the xanthate group from various synthetic targets that do not contain sulfur. The process involves heating xanthates in 2-propanol in the presence of equimolar amounts of dilauroyl peroxide, which is added in small portions. This method is advantageous because it does not require high dilution or slow addition of reagents, unlike traditional stannane-based radical reactions. The study concludes that this approach is efficient, particularly for secondary xanthates, and demonstrates tolerance to a variety of functional groups commonly encountered in organic synthesis. However, limitations were observed with primary xanthates, where large amounts of peroxide were needed and yields were poor.
10.1016/j.tetlet.2004.04.111
The study presents a new method for the ortho-substitution of anilines and the synthesis of indolines. The process begins with a radical addition of a xanthate to a vinyl sulfanilide, leading to the formation of a dihydrobenzoisothiazole dioxide structure. This intermediate loses sulfur dioxide upon heating to yield a 2-substituted aniline. In some instances, the presence of DBU during heating induces the formation of an indoline. The vinyl sulfanilides are prepared from 2-chloroethylsulfonyl chloride and anilines. The xanthates used in the radical addition can be benzylic, tertiary, or substituted with electrophilic groups like nitriles, ketones, or ketoesters. The study explores various substituents on the nitrogen and the aromatic ring, finding that electron-withdrawing groups facilitate the formation of indolines. The method is notable for its use of readily available starting materials and reagents, and its potential to access compounds that are difficult to obtain through classical approaches.