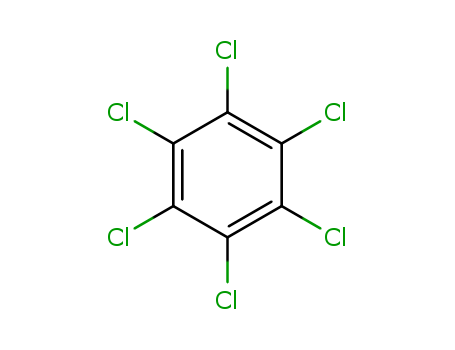
- Chemical Name:Hexachlorobenzene
- CAS No.:118-74-1
- Deprecated CAS:1135443-45-6
- Molecular Formula:C6Cl6
- Molecular Weight:284.784
- Hs Code.:2903920000
- European Community (EC) Number:204-273-9
- ICSC Number:0895
- NSC Number:9243
- UN Number:2729
- UNII:4Z87H0LKUY
- DSSTox Substance ID:DTXSID2020682
- Nikkaji Number:J2.911D
- Wikipedia:Hexachlorobenzene
- Wikidata:Q409682
- NCI Thesaurus Code:C44388
- Metabolomics Workbench ID:74829
- ChEMBL ID:CHEMBL228514
- Mol file:118-74-1.mol
Synonyms:HCB;Hexachlorobenzene


 T,
T, N
N


