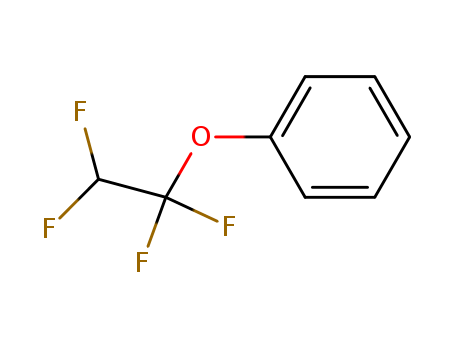
- Chemical Name:(1,1,2,2-Tetrafluoroethoxy)benzene
- CAS No.:350-57-2
- Molecular Formula:C8H6F4O
- Molecular Weight:194.129
- Hs Code.:29093090
- European Community (EC) Number:206-505-4
- DSSTox Substance ID:DTXSID00188534
- Nikkaji Number:J193.818E
- Wikidata:Q83060333
- Mol file:350-57-2.mol
Synonyms:(1,1,2,2-Tetrafluoroethoxy)benzene;350-57-2;1,1,2,2-tetrafluoroethoxybenzene;Fentalene-14;Benzene, (1,1,2,2-tetrafluoroethoxy)-;EINECS 206-505-4;BRN 2255899;C8H6F4O;alpha,alpha,beta,beta-Tetrafluorophenetole;(Tetrafluoroethoxy)benzene;3-06-00-00598 (Beilstein Handbook Reference);Benzene, (tetrafluoroethoxy)-;SCHEMBL194052;Ether, phenyl tetrafluoroethoxy;Tetrafluoroethyl ether of phenol;NIOSH/DC0300000;DTXSID00188534;C8-H6-F4-O;HMS1788M22;MFCD00042102;AKOS001116768;4-(1,1,2,2-tetrafluoroethoxy)benzene;(1,1,2,2-Tetrafluoro-ethoxy)-benzene;LS-32177;LS-32178;PS-11516;(1,1,2,2-Tetrafluoroethoxy)benzene, 97%;DC03000000;FT-0604490;EN300-18672;F81508;J-019845


 Xi,
Xi, T
T


