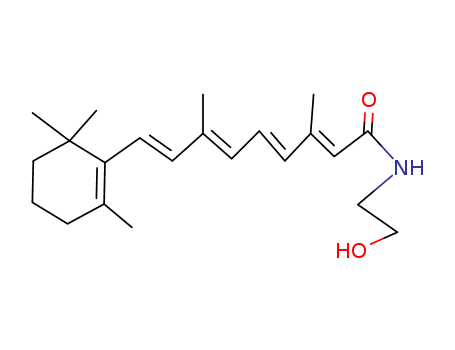
- Chemical Name:N-(2-Hydroxyethyl)retinamide
- CAS No.:33631-47-9
- Molecular Formula:C22H33NO2
- Molecular Weight:343.51
- Hs Code.:
- UNII:ZBJ8HC23XW
- DSSTox Substance ID:DTXSID801309774
- Nikkaji Number:J394.885D
- Pharos Ligand ID:YQUMGD7ZM1AX
- ChEMBL ID:CHEMBL60405
- Mol file:33631-47-9.mol
Synonyms:N-(2-hydroxyethyl)retinamide;N-(2-hydroxyethyl)retinamide, (13-cis)-isomer