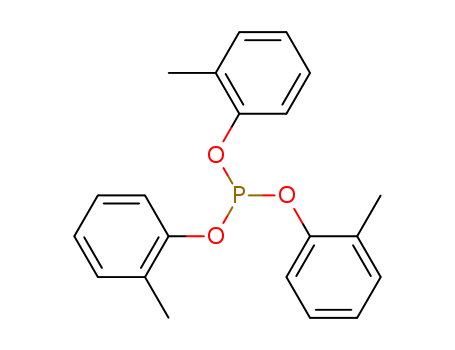
- Chemical Name:Tri-o-tolyl phosphite
- CAS No.:2622-08-4
- Molecular Formula:C21H21 O3 P
- Molecular Weight:352.37
- Hs Code.:2920901900
- European Community (EC) Number:220-068-7
- UNII:80ZL69270G
- DSSTox Substance ID:DTXSID40881239
- Nikkaji Number:J50.502A
- Wikidata:Q26841192
- Mol file:2622-08-4.mol
Synonyms:Tri-o-tolyl phosphite;2622-08-4;Tris(2-methylphenyl) phosphite;Tri-o-cresyl phosphite;Phosphorous Acid Tri-o-cresyl Ester;Tris(o-methylphenyl) phosphite;Tris(o-tolyloxy)phosphine;Tris(2-tolyl)phosphite;Phosphorous acid, tris(2-methylphenyl) ester;Phosphorous acid, tri-o-cresyl ester;EINECS 220-068-7;80ZL69270G;tri-o-Tolylphosphite;Phosphorous Acid Tris(2-methylphenyl) Ester;tri-tolylphosphite;trio-tolyl phosphite;tri(o-tolyl)phosphite;O-TOLYL PHOSPHITE;tris(methylphenyl)phosphite;tri(2-methylphenyl)phosphite;SCHEMBL215848;UNII-80ZL69270G;DTXSID40881239;Tris(2-methylphenyl) phosphite #;Phosphorous Acid Tri-o-tolyl Ester;CAA62208;MFCD00014911;TRIS(2-METHYLPHENOXY)PHOSPHINE;AKOS015899592;O-TOLYL PHOSPHITE ((C7H7O)3P);AS-66177;LS-109018;PHOSPHOROUS ACID, TRI-O-TOLYL ESTER;CS-0323839;P1416;F19565;Q26841192


 Xi
Xi


