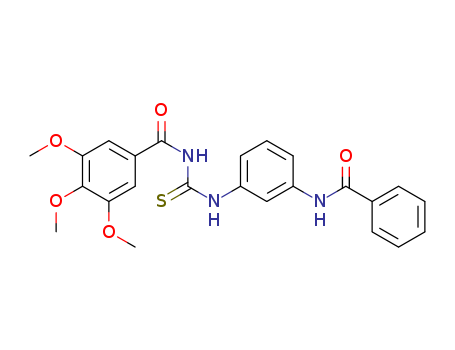
- Chemical Name:N-[[[3-[(Benzoyl)amino]phenyl]amino](thioxo)methyl]-3,4,5-trimethoxybenzamide
- CAS No.:330829-30-6
- Molecular Formula:C24H23N3O5S
- Molecular Weight:465.52
- Hs Code.:
- European Community (EC) Number:694-371-1
- DSSTox Substance ID:DTXSID90980483
- Wikidata:Q82966439
- ChEMBL ID:CHEMBL2031084
- Mol file:330829-30-6.mol
Synonyms:MRT 10;330829-30-6;MRT-10;N-[[[3-[(Benzoyl)amino]phenyl]amino](thioxo)methyl]-3,4,5-trimethoxybenzamide;N-[(3-Benzamidophenyl)carbamothioyl]-3,4,5-trimethoxybenzamide;CHEMBL2031084;6384-24-3;N-((3-Benzamidophenyl)carbamothioyl)-3,4,5-trimethoxybenzamide;Oprea1_307096;SCHEMBL12129860;DTXSID90980483;n-(3-benzamidophenylcarbamothioyl)-3,4,5-trimethoxybenzamide;EX-A7242;BDBM50383034;STK099293;AKOS000619111;NCGC00386664-02;MRT-10, >=98% (HPLC);MS-28575;HY-108507;CS-0029030;E98716;AG-690/15430158;J-019016;N-[[[3-Benzoylamino)phenyl]amino]thioxomethyl]-3,4,5-trimethoxybenzamide;N-[3-({[(3,4,5-trimethoxybenzoyl)amino]carbothioyl}amino)phenyl]benzamide;3,4,5-trimethoxy-N-({3-[(phenylcarbonyl)amino]phenyl}carbamothioyl)benzamide;N-{[(3-{[Hydroxy(phenyl)methylidene]amino}phenyl)imino](sulfanyl)methyl}-3,4,5-trimethoxybenzene-1-carboximidic acid