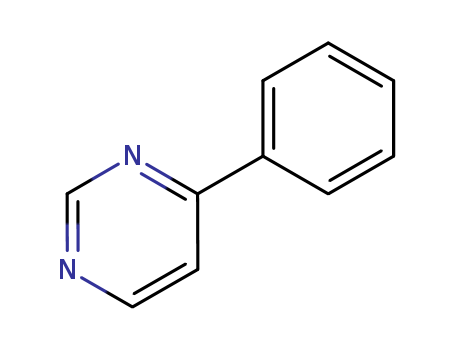
- Chemical Name:4-Phenylpyrimidine
- CAS No.:3438-48-0
- Molecular Formula:C10H8N2
- Molecular Weight:156.187
- Hs Code.:29335990
- European Community (EC) Number:222-345-8
- NSC Number:84249
- UNII:VQR91IRU2R
- DSSTox Substance ID:DTXSID90187950
- Nikkaji Number:J86.946E
- Wikidata:Q27162633
- ChEMBL ID:CHEMBL1235765
- Mol file:3438-48-0.mol
Synonyms:4-Phenylpyrimidine;3438-48-0;PYRIMIDINE, 4-PHENYL-;6-Phenylpyrimidine;4-phenyl-pyrimidine;EINECS 222-345-8;NSC 84249;VQR91IRU2R;BRN 0112781;MLS002694538;CHEBI:90622;NSC-84249;5-23-08-00009 (Beilstein Handbook Reference);NSC84249;RW1;3b9s;UNII-VQR91IRU2R;4-Phenylpyrimidine, 96%;SCHEMBL2131373;CHEMBL1235765;MKLQPIYLZMLAER-UHFFFAOYSA-;DTXSID90187950;HMS3094K17;AMY11943;MFCD00006111;AKOS015917050;DS-6698;SB57256;SMR001560464;LS-135512;CS-0060519;FT-0634855;P2525;EN300-130842;AC-907/25014265;J-019578;Q27162633


 Xi
Xi


