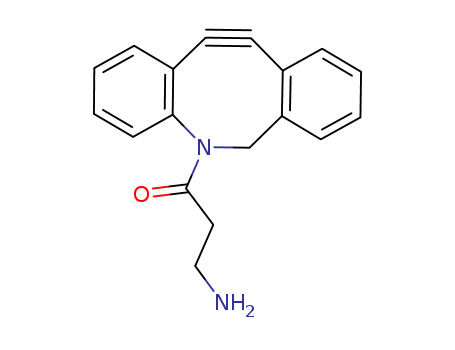
Suppliers and Price of Dibenzocyclooctyne-aMine for Copper-free Click CheMistry
- Business phase:
- The product has achieved commercial mass production*data from LookChem market partment
- Manufacturers and distributors:
-
- Manufacture/Brand
- Chemicals and raw materials
- Packaging
- price
- TRC
- DBCO-Amine
- 5mg
- $ 120.00
- TRC
- DBCO-Amine
- 50mg
- $ 900.00
- Sigma-Aldrich
- Dibenzocyclooctyne-amine for Copper-free Click Chemistry
- 10mg
- $ 51.60
- Sigma-Aldrich
- Dibenzocyclooctyne-amine for Copper-free Click Chemistry
- 100mg
- $ 422.00
- Iris Biotech GmbH
- DBCO-NH2
- 500 mg
- $ 945.00
- Iris Biotech GmbH
- DBCO-NH2
- 100 mg
- $ 270.00
- dPEG
- DBCO-amine
- 100 mg
- $ 240.00
- Crysdot
- DIBAC 97%
- 250mg
- $ 441.00
- Crysdot
- DIBAC 97%
- 1g
- $ 1112.00
- Chem-Impex
- DBCOamine,99%(HPLC) 99%(HPLC)
- 1G
- $ 1624.00
-
Total 36 raw suppliers