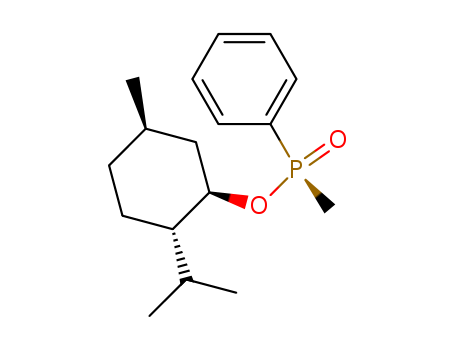
- Chemical Name:Methylphenylphosphinic acid (1R,3R,4S)-p-menthane-3-yl ester
- CAS No.:16934-92-2
- Molecular Formula:C17H27O2P
- Molecular Weight:294.374
- Hs Code.:
- Mol file:16934-92-2.mol
Synonyms:(-)-Methylphenylphosphinic acid (1R)-5β-methyl-2α-(1-methylethyl)cyclohexane-1β-yl ester;(+)-Methylphenylphosphinic acid (1R)-5β-methyl-2α-(1-methylethyl)cyclohexane-1β-yl ester;Methylphenylphosphinic acid (1R,3R,4S)-p-menthane-3-yl ester