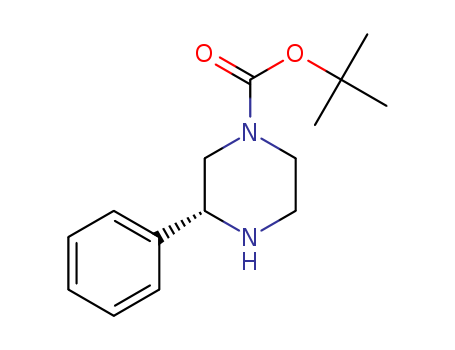
- Chemical Name:(R)-Tert-butyl 3-phenylpiperazine-1-carboxylate
- CAS No.:1240584-34-2
- Molecular Formula:C15H22N2O2
- Molecular Weight:262.352
- Hs Code.:2933599590
- European Community (EC) Number:835-718-0,949-005-8
- DSSTox Substance ID:DTXSID80426749
- Nikkaji Number:J3.107.024D
- Wikidata:Q72498209
- ChEMBL ID:CHEMBL5008993
- Mol file:1240584-34-2.mol
Synonyms:1240584-34-2;(R)-TERT-BUTYL 3-PHENYLPIPERAZINE-1-CARBOXYLATE;tert-butyl (3R)-3-phenylpiperazine-1-carboxylate;tert-Butyl (R)-3-phenylpiperazine-1-carboxylate;R-4-Boc-2-phenylpiperazine;(R)-3-PHENYL-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER;(R)-4-Boc-2-phenylpiperazine;rel-tert-butyl (3R)-3-phenylpiperazine-1-carboxylate;(R)-1-Boc-3-Phenylpiperazine;CHEMBL5008993;DTXSID80426749;MFCD08685942;1-Piperazinecarboxylic acid, 3-phenyl-, 1,1-dimethylethyl ester, (3R)-;AKOS022186376;TS-03580;CS-0091687;t-Butyl (R)-3-phenylpiperazine-1-carboxylate;EN300-6501354;EN300-6746236;(R)-TERT-BUTYL3-PHENYLPIPERAZINE-1-CARBOXYLATE;(3R)-3beta-Phenylpiperazine-1-carboxylic acid tert-butyl ester