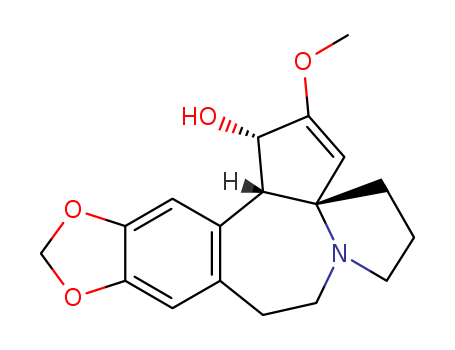
- Chemical Name:(2S,6S)-4-Methoxy-16,18-dioxa-10-azapentacyclo[11.7.0.02,6.06,10.015,19]icosa-1(20),4,13,15(19)-tetraen-3-one
- CAS No.:24316-19-6
- Molecular Formula:C18H21NO4
- Molecular Weight:315.369
- Hs Code.:
- DSSTox Substance ID:DTXSID3048231
- Nikkaji Number:J132.872G
- Wikidata:Q105289075
- Mol file:24316-19-6.mol
Synonyms:(2S,6S)-4-Methoxy-16,18-dioxa-10-azapentacyclo[11.7.0.02,6.06,10.015,19]icosa-1(20),4,13,15(19)-tetraen-3-one;Cephalotaxine, 3-deoxy-3-oxo-;3-Deoxy-3-oxocephalotaxine;SCHEMBL3761328;DTXSID3048231;38750-57-1


 Xn
Xn


