10.1021/jo00143a029
The research focuses on the synthesis of 2-(substituted phenyl) 2-oxazolines, which are key intermediates in the development of pharmaceutically active unsymmetrically disubstituted tetrahydroisoquinolines. The study explores the cross-coupling of alkyl and aryl Grignard reagents with 2-(mono- and dihalogenated phenyl) 2-oxazolines under nickel-phosphine complex catalysis, observing regioselective cross-coupling with 2-(dihalogenated phenyl) 2-oxazolines when a halogen is ortho to the 2-oxazolinyl moiety. The purpose of this research is to develop a two-step synthesis method for tetrahydroisoquinolines using 2-(disubstituted phenyl) 2-oxazolines as intermediates.
10.1039/b926965g
The research presents a concise total synthesis of PDE-II, a compound with inhibitory activity towards cyclic adenosine-3',5'-monophosphate phosphodiesterase, using a copper-mediated double aryl amination method. The purpose of this study is to develop an efficient and straightforward route for synthesizing PDE-II, given its importance as a partial structure of potent DNA alkylating agents like CC-1065 and yatakemycin. The synthesis involves a one-pot five-step sequence, starting from tetrahydroisoquinoline 7, and includes steps such as transacetalization, introduction of a glycine moiety, double aryl amination, and selective reduction. The study concludes that the developed method allows for the synthesis of PDE-II in 7.5% yield over 11 steps, demonstrating a significant improvement in efficiency compared to previous methods. The findings suggest that this synthetic strategy could be generally applicable to a wide variety of nitrogen-containing heterocycles due to the high utility of the aryl amination reaction.
10.1039/jr9530004089
The study investigates the reaction of hydrogen sulphide and formaldehyde with aromatic amines such as aniline and p-toluidine. The researchers found that these amines can condense with hydrogen sulphide and formaldehyde to form various compounds, including tetrahydrothiadiazines, thia-azetidines, and dihydrodithiazines. The specific products formed depend on the proportions of the reactants used. For instance, when 1 mol of hydrogen sulphide in aqueous-ethanolic formaldehyde is condensed with 2 mols of the amine, tetrahydrothiadiazines are obtained. However, when 2 mols of hydrogen sulphide are condensed with 1 mol of amine, mixtures of thia-azetidines and dihydrodithiazines are produced. The study also notes that all the cyclic products rapidly decompose to trithioformaldehyde and the amine hydrochloride when heated with hydrochloric acid.
10.1016/j.tetlet.2010.09.149
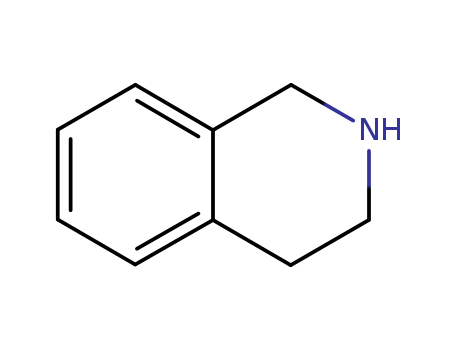
The study explores the use of o-benzenedisulfonimide as a reusable Br?nsted acid catalyst in the Pictet–Spengler reaction to synthesize tetrahydroisoquinolines and tetrahydro-β-carbolines. These compounds possess various biological activities and are found in numerous natural and synthetic organic compounds. The reaction involves 2-arylethylamine derivatives and aldehydes, with o-benzenedisulfonimide facilitating the cyclization of iminium ions formed from the dehydration reaction of these reactants. The study demonstrates that the catalyst can be easily recovered and reused, offering economic and ecological benefits. The reactions are carried out under mild and green conditions, yielding good target product yields.
10.1016/j.tetlet.2021.152873
The research focuses on the development of an efficient synthetic approach to access functionalized dihydro-[1,3]oxazino[4,3-a] isoindole and tetrahydroisoquinoline skeletons, which are important structural motifs in pharmaceuticals. The key experiments involve the addition-cyclization process of ynamides with N-acyliminium ions generated from N,O-acetals, catalyzed by Cu(OTf)2. A variety of substituted dihydro-[1,3]oxazino[4,3-a] isoindoles and tetrahydroisoquinolines were synthesized with yields ranging from 48% to 98%. The study also explored the use of chiral ynamides, leading to optically pure products with good to excellent yields and diastereoselectivities. The analyses used to confirm the chemical structures of the synthesized compounds included X-ray crystallographic analysis and ECD calculations, which were crucial for determining the absolute configurations of the new stereogenic centers.
10.1016/j.ejmech.2007.09.009
The study focuses on the design, synthesis, and evaluation of seventeen tetrahydroisoquinoline derivatives for their ability to inhibit nitric oxide (NO) production in lipopolysaccharide-stimulated BV-2 microglial cells. These derivatives were synthesized to potentially control inflammation-related damages by targeting the overproduction of NO, which is implicated in various diseases such as stroke, Alzheimer’s, and Parkinson’s. The chemicals used in the study include L-arginine, nitric oxide synthase (NOS) subtypes, tetrahydrobiopterin (BH4), and a series of tetrahydroisoquinoline derivatives with variable alkyl and acyl groups at different positions. The purpose of these chemicals was to investigate their inhibitory effects on NO production and to understand the structure-activity relationships, with the aim of identifying potent compounds that can reduce NO and BH4 production effectively. The study found that certain derivatives, particularly N-ethylcarbonyl-7-hydroxy-6-methoxy-1,2,3,4-tetrahydroisoquinoline (11a), showed a significant reduction in NO and BH4 production, suggesting their potential as therapeutic candidates for diseases associated with NO overproduction.
10.1016/j.tetlet.2014.07.043
The study presents an optimized biomimetic method for the conversion of various α-amino acids to aldehydes using sodium hypochlorite (NaOCl), which serves as an oxidizing agent for the decarboxylation of amino acids. The aldehyde products can then be transformed into tetrahydroisoquinolines, either racemic or (S)-enantiomer forms, through the Pictet–Spengler reaction with dopamine. The study utilizes a phosphate buffer to maximize regioselectivity for the racemic products and a maleic acid buffer for the enantioselective enzymatic synthesis of (S)-enantiomer products using norcoclaurine synthase. The purpose of these chemicals is to facilitate the synthesis of tetrahydroisoquinolines, which are found in numerous natural products and synthetic compounds with pharmacological activity, including benzoisoquinoline alkaloids. The study aims to synthesize novel BIAs with potential pharmacological utility by employing precursor-directed biosynthesis, avoiding the need for chromatography and ensuring the preparations are free of toxic chemical species.
10.1055/s-1982-29848
The research describes a novel variant of the internal α-amidoalkylation reaction, which is a method for synthesizing different heterocycles using compounds with amidoalkyl-electrophile and carbon-nucleophile centers within the same molecule. The purpose of this study was to develop a new approach to synthesize 1-aryl-3-oxo-2,3-dihydroisoquinolines, which are structurally significant and useful for the synthesis of tetrahydroisoquinolines. The researchers used arylacetic acids and secondary arylamides as starting compounds, and the reaction was carried out by heating these in equimolar quantities under reflux in phosphoryl chloride for 5 hours. The resulting products were 1-aryl-3-oxo-2,3-dihydroisoquinolines, which were yellow to red colored compounds. The structure of one of the products, 7a, was confirmed by its reduction with sodium borohydride and lithium alanate, yielding specific tetrahydroisoquinolines. The study concluded that the reaction proceeds via arylacetyl chlorides and imidoyl chlorides as probable intermediates, thus being an internal α-amidoalkylation process.


 Xi
Xi

