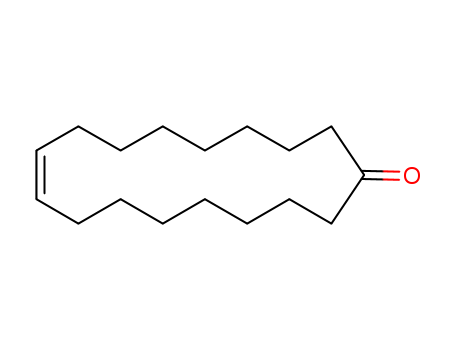- Chemical Name:Civetone
- CAS No.:542-46-1
- Molecular Formula:C17H30O
- Molecular Weight:250.425
- Hs Code.:2914299000
- European Community (EC) Number:208-813-4
- NSC Number:90305
- UNII:P0K30CV1UE
- DSSTox Substance ID:DTXSID80883434
- Nikkaji Number:J57.867C,J6.400I
- Wikipedia:Civetone
- Wikidata:Q198354
- Mol file:542-46-1.mol
Synonyms:9-cycloheptadecen-1-one;civetone;civetone, (Z)-isomer