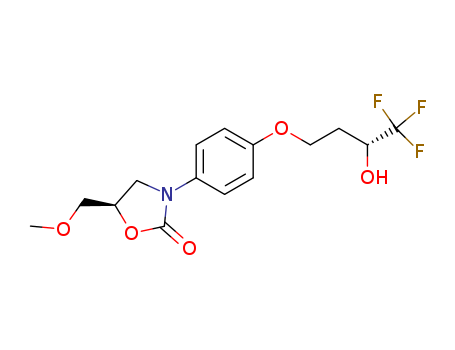
- Chemical Name:Befloxatone
- CAS No.:134564-82-2
- Molecular Formula:C15H18F3NO5
- Molecular Weight:349.307
- Hs Code.:
- UNII:4H75PAD8M3
- DSSTox Substance ID:DTXSID70158800
- Nikkaji Number:J561.796K
- Wikipedia:Befloxatone
- Wikidata:Q4880243
- NCI Thesaurus Code:C77519
- Metabolomics Workbench ID:144621
- ChEMBL ID:CHEMBL416578
- Mol file:134564-82-2.mol
Synonyms:befloxatone;MD 370,503;MD 370503;MD-370,503;MD-370503;MD370503