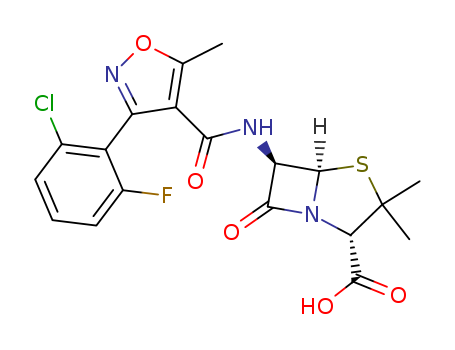
Chemical Property of Flucloxacillin
Edit
Chemical Property:
- Appearance/Colour:white
to off white free flowing crystalline powder
- Vapor Pressure:2.86E-19mmHg at 25°C
- Boiling Point:677.3 °C at 760 mmHg
- PKA:pKa 2.7 (Uncertain)
- Flash Point:363.4 °C
- PSA:138.04000
- Density:1.59 g/cm3
- LogP:3.01660
- XLogP3:2.6
- Hydrogen Bond Donor Count:2
- Hydrogen Bond Acceptor Count:8
- Rotatable Bond Count:4
- Exact Mass:453.0561477
- Heavy Atom Count:30
- Complexity:758
- Purity/Quality:
-
99% *data from raw suppliers
Flucloxacillin *data from reagent suppliers
Safty Information:
- Pictogram(s):
UN
NO.
- Hazard Codes:UN
NO.
- MSDS Files:
-
Useful:
- Canonical SMILES:CC1=C(C(=NO1)C2=C(C=CC=C2Cl)F)C(=O)NC3C4N(C3=O)C(C(S4)(C)C)C(=O)O
- Isomeric SMILES:CC1=C(C(=NO1)C2=C(C=CC=C2Cl)F)C(=O)N[C@H]3[C@@H]4N(C3=O)[C@H](C(S4)(C)C)C(=O)O
- Recent ClinicalTrials:Penicillin Against Flucloxacillin Treatment Evaluation
- Recent EU Clinical Trials:Flucloxacillin as an inducer of CYP-enzymes
-
Description
Chemically this is 3(2-chloro-6-fluorophenyl)-5-methyl-4-isoxazolyl
penicillin; this differs from dicloxacillin only by the substitution of a fluorine for a chlorine atom (Sutherland et al., 1970). It comes as oral
capsules of 250 and 500 mg, as a suspension of 25 and 50 mg/ml, and in
an injectable formulation of 500 mg and 1 g.
-
Uses
Antibacterial.
-
Therapeutic Function
Antibacterial
-
Clinical Use
Uses are those of group 3 penicillins.
-
Drug interactions
Potentially hazardous interactions with other drugs
Reduces excretion of methotrexate.