10.1021/jo00304a024
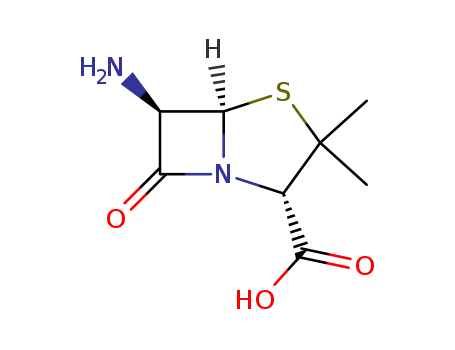
The research focuses on the stereocontrolled total syntheses of penicillanic acid S,S-dioxide (10) and 6-aminopenicillanic acid (26) derived from (S)-aspartic acid and (R,R)-tartaric acid, respectively. The study's key steps involve the preparation and cyclization of nitroalkenes 8 and 23, with the reaction of these compounds with tetrabutylammonium fluoride followed by ozone and DBU yielding the bicyclic P-lactams 9 and 24, which are then transformed into the target penicillanic acid derivatives 10 and 26. The research concludes that (benzyloxy)nitromethane is a highly useful reagent in β-lactam chemistry, and the nitroalkene ring closure strategy is efficient and effective for preparing polyfunctional bicyclic β-lactams, with potential general applicability for constructing novel β-lactam systems.


 Xn
Xn


